Loxapine - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for loxapine and what is the scope of patent protection?
Loxapine
is the generic ingredient in five branded drugs marketed by Alexza Pharms, Teva Branded Pharm, Actavis Labs Ut Inc, Elite Labs Inc, Lannett Co Inc, Rising, and Watson Labs, and is included in eleven NDAs. There is one patent protecting this compound. Additional information is available in the individual branded drug profile pages.Loxapine has twenty-one patent family members in six countries.
There are eight drug master file entries for loxapine.
Summary for loxapine
| International Patents: | 21 |
| US Patents: | 1 |
| Tradenames: | 5 |
| Applicants: | 7 |
| NDAs: | 11 |
| Drug Master File Entries: | 8 |
| Raw Ingredient (Bulk) Api Vendors: | 52 |
| Clinical Trials: | 26 |
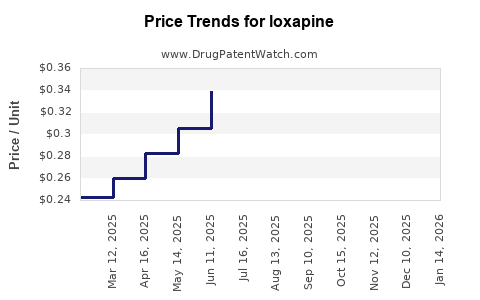
| Drug Prices: | Drug price trends for loxapine |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for loxapine |
| What excipients (inactive ingredients) are in loxapine? | loxapine excipients list |
| DailyMed Link: | loxapine at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for loxapine
Generic Entry Date for loxapine*:
Constraining patent/regulatory exclusivity:
Dosage:
POWDER;INHALATION |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for loxapine
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University Hospital, Strasbourg, France | PHASE2 |
| Lariboisière-Saint Louis clinical research unit | Phase 3 |
| Assistance Publique - Hôpitaux de Paris | Phase 3 |
Medical Subject Heading (MeSH) Categories for loxapine
Anatomical Therapeutic Chemical (ATC) Classes for loxapine
US Patents and Regulatory Information for loxapine
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elite Labs Inc | LOXAPINE SUCCINATE | loxapine succinate | CAPSULE;ORAL | 076868-003 | Aug 4, 2005 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Rising | LOXAPINE SUCCINATE | loxapine succinate | CAPSULE;ORAL | 076762-004 | Nov 1, 2004 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Teva Branded Pharm | LOXITANE C | loxapine hydrochloride | CONCENTRATE;ORAL | 017658-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Watson Labs | LOXAPINE SUCCINATE | loxapine succinate | CAPSULE;ORAL | 072062-001 | Jun 15, 1988 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Rising | LOXAPINE SUCCINATE | loxapine succinate | CAPSULE;ORAL | 076762-003 | Nov 1, 2004 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for loxapine
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Alexza Pharms | ADASUVE | loxapine | POWDER;INHALATION | 022549-001 | Dec 21, 2012 | 7,585,493 | ⤷ Get Started Free |
| Alexza Pharms | ADASUVE | loxapine | POWDER;INHALATION | 022549-001 | Dec 21, 2012 | 9,440,034 | ⤷ Get Started Free |
| Alexza Pharms | ADASUVE | loxapine | POWDER;INHALATION | 022549-001 | Dec 21, 2012 | 6,716,416 | ⤷ Get Started Free |
| Alexza Pharms | ADASUVE | loxapine | POWDER;INHALATION | 022549-001 | Dec 21, 2012 | 8,235,037 | ⤷ Get Started Free |
| Alexza Pharms | ADASUVE | loxapine | POWDER;INHALATION | 022549-001 | Dec 21, 2012 | 8,173,107 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for loxapine
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Ferrer Internacional S.A. | Adasuve | loxapine | EMEA/H/C/002400Adasuve is indicated for the rapid control of mild-to-moderate agitation in adult patients with schizophrenia or bipolar disorder. Patients should receive regular treatment immediately after control of acute agitation symptoms. | Authorised | no | no | no | 2013-02-20 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for loxapine
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Canada | 2526478 | PROCEDE DE REGULATION THERMIQUE UNIFORME D'UN SUBSTRAT ; UNITE DE CHAUFFAGE INTEGREE ET UNITE D'ADMINISTRATION DE MEDICAMENTS RECOURANT A CE PROCEDE (METHODS OF CONTROLLING UNIFORMITY OF SUBSTRATE TEMPERATURE AND SELF-CONTAINED HEATING UNIT AND DRUG-SUPPLY UNIT EMPLOYING SAME) | ⤷ Get Started Free |
| European Patent Office | 2096374 | Unité de chauffage autonome et unité d'administration d'un médicament utilisant une telle unité de chauffage (Self-contained heating unit and drug-supply unit employing same) | ⤷ Get Started Free |
| Austria | 520935 | ⤷ Get Started Free | |
| Japan | 2007516149 | ⤷ Get Started Free | |
| Canada | 2526432 | UNITE DE CHAUFFAGE AUTONOME ET UNITE DE FOURNITURE DE MEDICAMENT FAISANT APPEL A CETTE UNITE DE CHAUFFAGE (SELF-CONTAINED HEATING UNIT AND DRUG-SUPPLY UNIT EMPLOYING SAME) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for loxapine
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1389098 | 300609 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: LOXAPINE; REGISTRATION NO/DATE: EU/1/13/823/001-002 20130220 |
| 1389098 | 2013C/054 | Belgium | ⤷ Get Started Free | PRODUCT NAME: LOXAPINE; AUTHORISATION NUMBER AND DATE: EU/1/13/823/001 20130225 |
| 1389098 | C300609 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: LOXAPINE; REGISTRATION NO/DATE: EU/1/13/823/001-002 20130220 |
| 1389098 | SPC/GB13/055 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: LOXAPINE; REGISTERED: UK EU/1/13/823/001 20130220; UK EU/1/13/823/002 20130220 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: Loxapine
More… ↓