Naltrexone - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for naltrexone and what is the scope of freedom to operate?
Naltrexone
is the generic ingredient in five branded drugs marketed by Teva Pharms Usa Inc, Alkermes, Accord Hlthcare, Barr, Chartwell, Elite Labs, Fosun Pharma, Specgx Llc, Sun Pharm, Teva Womens, and Pfizer, and is included in eleven NDAs. There are three patents protecting this compound. Additional information is available in the individual branded drug profile pages.Naltrexone has eighteen patent family members in eleven countries.
There are nineteen drug master file entries for naltrexone. One supplier is listed for this compound. There is one tentative approval for this compound.
Summary for naltrexone
| International Patents: | 18 |
| US Patents: | 3 |
| Tradenames: | 5 |
| Applicants: | 11 |
| NDAs: | 11 |
| Drug Master File Entries: | 19 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 46 |
| Clinical Trials: | 468 |
| Patent Applications: | 7,751 |
| Formulation / Manufacturing: | see details |
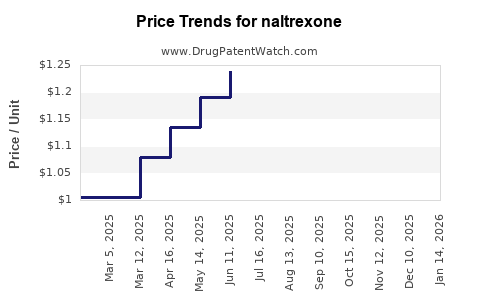
| Drug Prices: | Drug price trends for naltrexone |
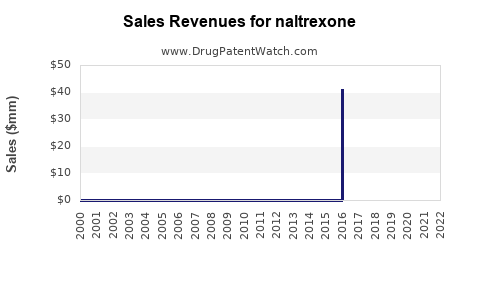
| Drug Sales Revenues: | Drug sales revenues for naltrexone |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for naltrexone |
| What excipients (inactive ingredients) are in naltrexone? | naltrexone excipients list |
| DailyMed Link: | naltrexone at DailyMed |
Recent Clinical Trials for naltrexone
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Inventage Lab., Inc. | Phase 1 |
| BioXcel Therapeutics Inc | Phase 1/Phase 2 |
| Bicycle Health | Phase 4 |
Generic filers with tentative approvals for NALTREXONE
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | 8MG/90MG | TABLET, EXTENDED RELEASE; ORAL |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Medical Subject Heading (MeSH) Categories for naltrexone
Paragraph IV (Patent) Challenges for NALTREXONE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| VIVITROL | Extended-release Injectable Suspension | naltrexone | 380 mg/vial | 021897 | 1 | 2020-06-18 |
US Patents and Regulatory Information for naltrexone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pfizer | TROXYCA ER | naltrexone hydrochloride; oxycodone hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 207621-004 | Aug 19, 2016 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Pfizer | TROXYCA ER | naltrexone hydrochloride; oxycodone hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 207621-006 | Aug 19, 2016 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Specgx Llc | NALTREXONE HYDROCHLORIDE | naltrexone hydrochloride | TABLET;ORAL | 076264-001 | Mar 22, 2002 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sun Pharm | NALTREXONE HYDROCHLORIDE | naltrexone hydrochloride | TABLET;ORAL | 090356-001 | Feb 24, 2012 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Specgx Llc | NALTREXONE HYDROCHLORIDE | naltrexone hydrochloride | TABLET;ORAL | 076264-003 | Mar 22, 2002 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for naltrexone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Alkermes | VIVITROL | naltrexone | FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 021897-001 | Apr 13, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Alkermes | VIVITROL | naltrexone | FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 021897-001 | Apr 13, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Alkermes | VIVITROL | naltrexone | FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 021897-001 | Apr 13, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Alkermes | VIVITROL | naltrexone | FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 021897-001 | Apr 13, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Alkermes | VIVITROL | naltrexone | FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 021897-001 | Apr 13, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for naltrexone
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Canada | 2563086 | PROCEDE ET SYSTEME D'INVESTIGATION ANONYME ETENDUE BASEE SURDES FAITS ET D'ETABLISSEMENT DE RAPPORTS A BASE DE FAITS ET D'ACCES SELECTIF AUX RESULTATS ET RAPPORTS (NALTREXONE LONG ACTING FORMULATIONS AND METHODS OF USE) | ⤷ Try a Trial |
| Russian Federation | 2370257 | СОСТАВЫ С ПРОЛОНГИРОВАННЫМ ДЕЙСТВИЕМ НА ОСНОВЕ НАЛТРЕКСОНА И СПОСОБЫ ИХ ПРИМЕНЕНИЯ (COMPOSITIONS WITH PROLONGED ACTION BASED ON NALTREXONE AND METHODS OF THEIR APPLICATION) | ⤷ Try a Trial |
| United Kingdom | 0304637 | ⤷ Try a Trial | |
| China | 103251597 | Naltrexone long acting formulations and methods of use | ⤷ Try a Trial |
| Japan | 5426094 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for naltrexone
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2316456 | 1790064-8 | Sweden | ⤷ Try a Trial | PRODUCT NAME: NALTREXONE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR NALTREXONE HYDROCHLORIDE, AND BUPROPION OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR BUPROPION HYDROCHLORIDE; REG. NO/DATE: EU/1/14/988 20150330 |
| 2316456 | 2017C/064 | Belgium | ⤷ Try a Trial | PRODUCT NAME: NALTREXONE/BUPROPION; AUTHORISATION NUMBER AND DATE: EU/1/14/988 20150330 |
| 2316456 | 2017/059 | Ireland | ⤷ Try a Trial | PRODUCT NAME: NALTREXONE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR NALTREXONE HYDROCHLORIDE, AND BUPROPION OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR BUPROPION HYDROCHLORIDE; REGISTRATION NO/DATE: EU/1/14/988 20150326 |
| 2316456 | 17C1058 | France | ⤷ Try a Trial | PRODUCT NAME: NALTREXONE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE,EN PARTICULIER CHLORHYDRATE DE NALTREXONE ET,BUPROPION OU SEL PHARMACEUTIQUEMENT ACCEPTABLE,EN PARTICULIER CHLORHYDRATE DE BUPROPION; REGISTRATION NO/DATE: EU/1/14/988 20150330 |
| 2316456 | SPC/GB17/078 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: NALTREXONE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR NALTREXONE HYDROCHLORIDE, AND BUPROPION OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR BUPROPION HYDROCHLORIDE.; REGISTERED: UK EU/1/14/988 20150330; UK PLGB 50742/0001 20150330 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |