TOBRAMYCIN - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for tobramycin and what is the scope of patent protection?
Tobramycin
is the generic ingredient in eleven branded drugs marketed by Novartis, Mylan Speciality Lp, Epic Pharma Llc, Alcon Pharms Ltd, Alembic, Apotex Inc, Bausch And Lomb, Chartwell Rx, Gland Pharma Ltd, Somerset Theraps Llc, Sandoz, Chiesi, Pulmoflow Inc, Alkem Labs Ltd, Amneal Pharms, Dr Reddys Labs Sa, Hikma, Luoxin Aurovitas, Lupin, Mankind Pharma, Micro Labs, Mylan, Sun Pharm, Teva Pharms Usa, Lilly, Apothecon, Baxter Hlthcare Corp, Eugia Pharma, Fresenius Kabi Usa, Hainan Poly, Hospira, Igi Labs Inc, Mylan Labs Ltd, Watson Labs Inc, Xellia Pharms Aps, and Xgen Pharms, and is included in sixty-four NDAs. There are seven patents protecting this compound and two Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Tobramycin has sixty-one patent family members in twenty-seven countries.
There are eighteen drug master file entries for tobramycin. Thirty-four suppliers are listed for this compound.
Summary for TOBRAMYCIN
| International Patents: | 61 |
| US Patents: | 7 |
| Tradenames: | 11 |
| Applicants: | 36 |
| NDAs: | 64 |
| Drug Master File Entries: | 18 |
| Finished Product Suppliers / Packagers: | 34 |
| Raw Ingredient (Bulk) Api Vendors: | 78 |
| Clinical Trials: | 121 |
| Patent Applications: | 6,924 |
| Formulation / Manufacturing: | see details |
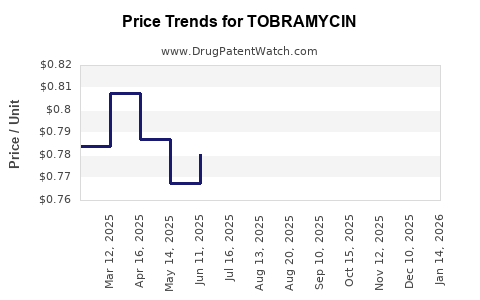
| Drug Prices: | Drug price trends for TOBRAMYCIN |
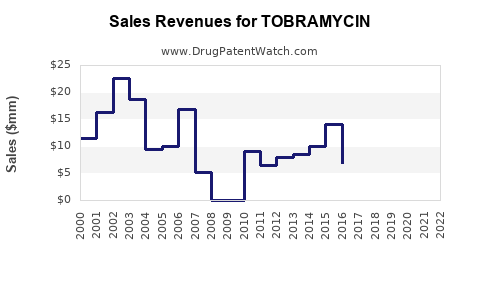
| Drug Sales Revenues: | Drug sales revenues for TOBRAMYCIN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TOBRAMYCIN |
| What excipients (inactive ingredients) are in TOBRAMYCIN? | TOBRAMYCIN excipients list |
| DailyMed Link: | TOBRAMYCIN at DailyMed |
Recent Clinical Trials for TOBRAMYCIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Utah | Early Phase 1 |
| University of Washington | Phase 4 |
| Medical University of South Carolina | Phase 4 |
Pharmacology for TOBRAMYCIN
| Drug Class | Aminoglycoside Antibacterial |
Medical Subject Heading (MeSH) Categories for TOBRAMYCIN
Paragraph IV (Patent) Challenges for TOBRAMYCIN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BETHKIS | Inhalation Solution | tobramycin | 300 mg/4 mL | 201820 | 1 | 2017-08-31 |
| TOBI | Inhalation Solution | tobramycin | 300 mg/5 mL | 050753 | 1 | 2009-06-29 |
US Patents and Regulatory Information for TOBRAMYCIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Igi Labs Inc | TOBRAMYCIN SULFATE | tobramycin sulfate | INJECTABLE;INJECTION | 063122-001 | Oct 31, 1994 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Gland Pharma Ltd | TOBRAMYCIN SULFATE | tobramycin sulfate | INJECTABLE;INJECTION | 211189-001 | May 18, 2021 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Xgen Pharms | TOBRAMYCIN SULFATE | tobramycin sulfate | INJECTABLE;INJECTION | 065013-001 | Aug 17, 2001 | AP | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Mankind Pharma | TOBRAMYCIN | tobramycin | SOLUTION;INHALATION | 214478-001 | Jun 30, 2023 | AN | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Gland Pharma Ltd | TOBRAMYCIN SULFATE | tobramycin sulfate | INJECTABLE;INJECTION | 209621-001 | Feb 11, 2021 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TOBRAMYCIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Mylan Speciality Lp | TOBI PODHALER | tobramycin | POWDER;INHALATION | 201688-001 | Mar 22, 2013 | ⤷ Try a Trial | ⤷ Try a Trial |
| Mylan Speciality Lp | TOBI PODHALER | tobramycin | POWDER;INHALATION | 201688-001 | Mar 22, 2013 | ⤷ Try a Trial | ⤷ Try a Trial |
| Mylan Speciality Lp | TOBI PODHALER | tobramycin | POWDER;INHALATION | 201688-001 | Mar 22, 2013 | ⤷ Try a Trial | ⤷ Try a Trial |
| Mylan Speciality Lp | TOBI PODHALER | tobramycin | POWDER;INHALATION | 201688-001 | Mar 22, 2013 | ⤷ Try a Trial | ⤷ Try a Trial |
| Mylan Speciality Lp | TOBI PODHALER | tobramycin | POWDER;INHALATION | 201688-001 | Mar 22, 2013 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for TOBRAMYCIN
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Viatris Healthcare Limited | Tobi Podhaler | tobramycin | EMEA/H/C/002155 Tobi Podhaler is indicated for the suppressive therapy of chronic pulmonary infection due to Pseudomonas aeruginosa in adults and children aged 6 years and older with cystic fibrosis. See sections 4.4 and 5.1 regarding data in different age groups.Consideration should be given to official guidance on the appropriate use of antibacterial agents. |
Authorised | no | no | no | 2011-07-20 | |
| Pari Pharma GmbH | Vantobra (previously Tobramycin PARI) | tobramycin | EMEA/H/C/005086 Vantobra is indicated for the management of chronic pulmonary infection due to Pseudomonas aeruginosa in patients aged 6 years and older with cystic fibrosis (CF).Consideration should be given to official guidance on the appropriate use of antibacterial agents. |
Authorised | no | no | no | 2019-02-18 | |
| Pari Pharma GmbH | Vantobra | tobramycin | EMEA/H/C/002633 Vantobra is indicated for the management of chronic pulmonary infection due to Pseudomonas aeruginosa in patients aged 6 years and older with cystic fibrosis (CF).Consideration should be given to official guidance on the appropriate use of antibacterial agents. |
Withdrawn | no | no | no | 2015-03-18 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for TOBRAMYCIN
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2006522663 | ⤷ Try a Trial | |
| South Korea | 20070026604 | METHODS OF TREATMENT OF ENDOBRONCHIAL INFECTIONS | ⤷ Try a Trial |
| Japan | 4943838 | ⤷ Try a Trial | |
| Norway | 20070253 | ⤷ Try a Trial | |
| New Zealand | 552047 | Method of treatment of endobronchial infections | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TOBRAMYCIN
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1273292 | C01273292/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: TOBRAMYCIN; REGISTRATION NO/DATE: SWISSMEDIC 58751 28.05.2009 |
| 1280520 | 14/2015 | Austria | ⤷ Try a Trial | PRODUCT NAME: TOBRAMYCIN ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON; REGISTRATION NO/DATE: EU/1/10/652/001-003 20110720 |
| 1280520 | 300722 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: TOBRAMYCINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: C(2011)5394 20110720 |
| 1280520 | C01280520/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: TOBRAMYCIN; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 60565 01.02.2012 |
| 1280520 | C300722 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: TOBRAMYCINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: C(2011)5394 20110720 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.