UPJOHN Company Profile
✉ Email this page to a colleague
What is the competitive landscape for UPJOHN, and when can generic versions of UPJOHN drugs launch?
UPJOHN has thirteen approved drugs.
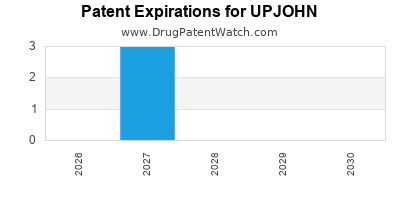
There are three US patents protecting UPJOHN drugs.
There are thirty-three patent family members on UPJOHN drugs in thirty-three countries and thirty-nine supplementary protection certificates in thirteen countries.
Drugs and US Patents for UPJOHN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upjohn | CELEBREX | celecoxib | CAPSULE;ORAL | 020998-001 | Dec 31, 1998 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Upjohn | CELEBREX | celecoxib | CAPSULE;ORAL | 020998-004 | Dec 15, 2006 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Upjohn | XANAX | alprazolam | TABLET;ORAL | 018276-002 | Approved Prior to Jan 1, 1982 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Upjohn | EFFEXOR XR | venlafaxine hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 020699-004 | Oct 20, 1997 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ||||
| Upjohn | XANAX | alprazolam | TABLET;ORAL | 018276-003 | Approved Prior to Jan 1, 1982 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ||||
| Upjohn | XANAX XR | alprazolam | TABLET, EXTENDED RELEASE;ORAL | 021434-001 | Jan 17, 2003 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for UPJOHN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-002 | Dec 17, 1996 | 5,686,104*PED | ⤷ Sign Up |
| Upjohn | XANAX | alprazolam | TABLET;ORAL | 018276-002 | Approved Prior to Jan 1, 1982 | 3,980,789 | ⤷ Sign Up |
| Upjohn | CELEBREX | celecoxib | CAPSULE;ORAL | 020998-003 | Aug 29, 2002 | 5,466,823*PED | ⤷ Sign Up |
| Upjohn | INSPRA | eplerenone | TABLET;ORAL | 021437-003 | Sep 27, 2002 | 6,495,165*PED | ⤷ Sign Up |
| Upjohn | CELEBREX | celecoxib | CAPSULE;ORAL | 020998-001 | Dec 31, 1998 | 5,563,165*PED | ⤷ Sign Up |
| Upjohn | XANAX | alprazolam | TABLET;ORAL | 018276-001 | Approved Prior to Jan 1, 1982 | 3,980,789 | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for UPJOHN drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Tablets | 82.5 mg and 165 mg | ➤ Subscribe | 2018-02-02 |
| ➤ Subscribe | Extended-release Tablets | 82.5 mg and 165 mg | ➤ Subscribe | 2018-02-02 |
| ➤ Subscribe | Tablets | 20 mg and 40 mg | ➤ Subscribe | 2010-03-29 |
| ➤ Subscribe | Extended-release Tablets | 37.5 mg, 75 mg and 150 mg | ➤ Subscribe | 2007-05-03 |
| ➤ Subscribe | Capsules | 25 mg, 50 mg, 75 mg, 100 mg, 150 mg, 200 mg, 225 mg and 300 mg | ➤ Subscribe | 2008-12-30 |
| ➤ Subscribe | Extended-release Tablets | 330 mg | ➤ Subscribe | 2018-01-29 |
| ➤ Subscribe | Extended-release Tablets | 330 mg | ➤ Subscribe | 2018-01-29 |
| ➤ Subscribe | Tablets | 25 mg and 50 mg | ➤ Subscribe | 2006-09-27 |
| ➤ Subscribe | Extended-release Capsules | 2 mg and 4 mg | ➤ Subscribe | 2007-07-30 |
| ➤ Subscribe | Capsules | 50 mg | ➤ Subscribe | 2008-03-21 |
| ➤ Subscribe | Oral Solution | 20 mg/mL | ➤ Subscribe | 2010-05-19 |
International Patents for UPJOHN Drugs
Supplementary Protection Certificates for UPJOHN Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0247633 | 97C0118 | France | ⤷ Sign Up | PRODUCT NAME: ATORVASTATINE CALCIQUE TRIHYDRATE; REGISTRATION NO/DATE IN FRANCE: NL 21960 DU 19970321; REGISTRATION NO/DATE AT EEC: PL 00018/0240 DU 19961107 |
| 0247633 | C970034 | Netherlands | ⤷ Sign Up | PRODUCT NAME: ATORVASTATINUM,DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT OF VAN HET INWENDIGE DELTA-LACTON, IN HET BIJZONDER ATORVASTATINUM CALCICUM TRIHYDRICUM; NAT. REGISTRATION NO/DATE: RVG 21081 - RVG 21083 19970421; FIRST REGISTRATION: GB PL 00018/0240 - PL 00018/0242 19961107 |
| 0122232 | 2004C/008 | Belgium | ⤷ Sign Up | PRODUCT NAME: EPLERENONE; NATIONAL REGISTRATION NO/DATE: 241 IS 188 F3 20041220; FIRST REGISTRATION: NL RVG 29963 20040316 |
| 0720599 | 122014000088 | Germany | ⤷ Sign Up | PRODUCT NAME: EZETIMIB ODER PHARMAZEUTISCH ANNEHMBARE SALZE DAVON IN KOMBINATION MIT ATORVASTATIN ODER PHARMAZEUTISCH ANNEHMBARE SALZE DAVON, INSBESONDERE ATORVASTATIN ALS ATORVASTATINCALCIUMTRIHYDRAT; NAT. REGISTRATION NO/DATE: 90223.00.00 20141113; FIRST REGISTRATION: FRANKREICH CIS: 6 928 917 6 20140912 |
| 0325571 | C980020 | Netherlands | ⤷ Sign Up | PRODUCT NAME: TOLTERODINE, DESGEWENST IN DE VORM VAN EEN ZOUT MET EEN FYSIOLO GISCH ACCEPTABEL ZUUR, IN HET BIJZONDER TOLTERODINE L-TARTRAAT; NAT. REGISTRATION NO/DATE: RVG 22148, RVG 22149 19980217; FIRST REGISTRATION: SE 13475, 13476 19970905 |
| 0364417 | SPC/GB97/014 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: LATANOPROST (I.E. 13,14-DIHYDRO-17-PHENYL-18,19,20-TRINOR-PGF-ALPHA-ISOPROPYLESTER); NAT. REGISTRATION NO/DATE: 00032/0220 19961216; FIRST REGISTRATION: SE 12716 19960718; SPC EXTENSION AUTHORISATION: PL00057/1057-008 20101216 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.