PURDUE PHARMA LP Company Profile
✉ Email this page to a colleague
What is the competitive landscape for PURDUE PHARMA LP, and what generic alternatives to PURDUE PHARMA LP drugs are available?
PURDUE PHARMA LP has nine approved drugs.
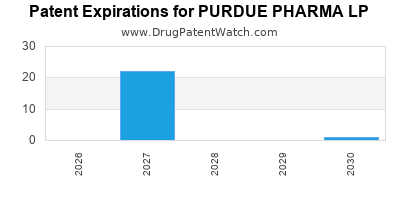
There are forty-two US patents protecting PURDUE PHARMA LP drugs.
There are four hundred and twenty-two patent family members on PURDUE PHARMA LP drugs in fifty-one countries and one supplementary protection certificate in one country.
Summary for PURDUE PHARMA LP
| International Patents: | 422 |
| US Patents: | 42 |
| Tradenames: | 9 |
| Ingredients: | 9 |
| NDAs: | 9 |
| Patent Litigation for PURDUE PHARMA LP: | See patent lawsuits for PURDUE PHARMA LP |
Drugs and US Patents for PURDUE PHARMA LP
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purdue Pharma Lp | TARGINIQ | naloxone hydrochloride; oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 205777-003 | Jul 23, 2014 | DISCN | Yes | No | 9,522,919 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Purdue Pharma Lp | OXYCONTIN | oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 022272-001 | Apr 5, 2010 | RX | Yes | No | 10,407,434 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Purdue Pharma Lp | OXYCONTIN | oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 022272-003 | Apr 5, 2010 | RX | Yes | No | 9,073,933 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Purdue Pharma Lp | ADHANSIA XR | methylphenidate hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 212038-004 | Feb 27, 2019 | DISCN | Yes | No | 10,722,473 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Purdue Pharma Lp | HYSINGLA ER | hydrocodone bitartrate | TABLET, EXTENDED RELEASE;ORAL | 206627-005 | Nov 20, 2014 | AB | RX | Yes | No | 9,486,413 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Purdue Pharma Lp | MS CONTIN | morphine sulfate | TABLET, EXTENDED RELEASE;ORAL | 019516-004 | Jan 16, 1990 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Purdue Pharma Lp | HYSINGLA ER | hydrocodone bitartrate | TABLET, EXTENDED RELEASE;ORAL | 206627-006 | Nov 20, 2014 | AB | RX | Yes | No | 9,492,389 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PURDUE PHARMA LP
Paragraph IV (Patent) Challenges for PURDUE PHARMA LP drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Transdermal System | 15 mcg/hr | ➤ Subscribe | 2013-12-16 |
| ➤ Subscribe | Extended-release Tablets | 30 mg, 40 mg, 80 mg, and 100 mg | ➤ Subscribe | 2015-05-08 |
| ➤ Subscribe | Extended-release Tablets | 30 mg and 60 mg | ➤ Subscribe | 2007-01-03 |
| ➤ Subscribe | Transdermal System | 5 mcg/hr, 10 mcg/hr, and 20 mcg/hr | ➤ Subscribe | 2013-06-06 |
| ➤ Subscribe | Extended-release Tablets | 20 mg, 60 mg, and 120 mg | ➤ Subscribe | 2015-04-15 |
| ➤ Subscribe | Extended-release Tablets | 15 mg | ➤ Subscribe | 2007-02-15 |
International Patents for PURDUE PHARMA LP Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Croatia | P20130434 | ⤷ Try a Trial |
| Denmark | 2292230 | ⤷ Try a Trial |
| South Korea | 20070022033 | ⤷ Try a Trial |
| Eurasian Patent Organization | 018311 | ⤷ Try a Trial |
| Poland | 1730151 | ⤷ Try a Trial |
| South Korea | 101205579 | ⤷ Try a Trial |
| Portugal | 2292229 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for PURDUE PHARMA LP Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1685839 | 92292 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON D OXYCODONE EN TANT QUE COMPOSANT A ET DE NALOXONE EN TANT QUE COMPOSANT B SOUS TOUTES LES FORMES PROTEGES PAR LE BREVET DE BASE |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.