PFIZER Company Profile
✉ Email this page to a colleague
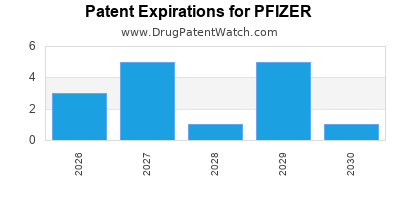
What is the competitive landscape for PFIZER, and when can generic versions of PFIZER drugs launch?
PFIZER has one hundred and ninety-five approved drugs.
There are forty-nine US patents protecting PFIZER drugs. There is one tentative approval on PFIZER drugs.
There are one thousand and twenty-seven patent family members on PFIZER drugs in seventy countries and two hundred and sixty-one supplementary protection certificates in nineteen countries.
Summary for PFIZER
| International Patents: | 1027 |
| US Patents: | 49 |
| Tradenames: | 159 |
| Ingredients: | 130 |
| NDAs: | 195 |
| Drug Master File Entries: | 3 |
| Patent Litigation for PFIZER: | See patent lawsuits for PFIZER |
| PTAB Cases with PFIZER as petitioner: | See PTAB cases with PFIZER as petitioner |
Drugs and US Patents for PFIZER
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pfizer | VELSIPITY | etrasimod arginine | TABLET;ORAL | 216956-001 | Oct 12, 2023 | RX | Yes | Yes | 11,007,175 | ⤷ Sign Up | ⤷ Sign Up | ||||
| Pfizer | CORTRIL | hydrocortisone acetate | INJECTABLE;INJECTION | 009164-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Pfizer | TALZENNA | talazoparib tosylate | CAPSULE;ORAL | 211651-005 | Jun 20, 2023 | RX | Yes | No | 8,735,392 | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Pfizer | IBRANCE | palbociclib | TABLET;ORAL | 212436-003 | Nov 1, 2019 | RX | Yes | Yes | RE47739 | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Pfizer | LONITEN | minoxidil | TABLET;ORAL | 018154-003 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Pfizer | VIZIMPRO | dacomitinib | TABLET;ORAL | 211288-003 | Sep 27, 2018 | RX | Yes | Yes | 8,623,883 | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PFIZER
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-002 | Dec 12, 2019 | 7,265,221 | ⤷ Sign Up |
| Pfizer | COVERA-HS | verapamil hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 020552-002 | Feb 26, 1996 | 5,200,196 | ⤷ Sign Up |
| Pfizer | MICRONASE | glyburide | TABLET;ORAL | 017498-002 | May 1, 1984 | 3,454,635 | ⤷ Sign Up |
| Pfizer | IBRANCE | palbociclib | CAPSULE;ORAL | 207103-003 | Feb 3, 2015 | 6,936,612 | ⤷ Sign Up |
| Pfizer | NICOTROL | nicotine | INHALANT;ORAL | 020714-001 | May 2, 1997 | 4,793,366 | ⤷ Sign Up |
| Pfizer | MINIZIDE | polythiazide; prazosin hydrochloride | CAPSULE;ORAL | 017986-002 | Approved Prior to Jan 1, 1982 | 3,511,836 | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for PFIZER drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 5 mg | ➤ Subscribe | 2016-11-07 |
| ➤ Subscribe | Delayed-release Tablets | 50 mg/0.2 mg | ➤ Subscribe | 2009-06-29 |
| ➤ Subscribe | Extended-release Tablets | 11 mg | ➤ Subscribe | 2016-11-07 |
| ➤ Subscribe | Capsules | 20 mg, 40 mg, 60 mg and 80 mg | ➤ Subscribe | 2005-02-07 |
| ➤ Subscribe | Capsules | 75 mg, 100 mg and 125 mg | ➤ Subscribe | 2019-02-04 |
| ➤ Subscribe | Capsules | 0.125 mg, 0.25 mg, and 0.5 mg | ➤ Subscribe | 2014-05-01 |
| ➤ Subscribe | Tablets | 600 mg | ➤ Subscribe | 2005-12-21 |
| ➤ Subscribe | Injection | 2 mg/mL, 300 mL bag | ➤ Subscribe | 2009-09-01 |
| ➤ Subscribe | Tablets | 1 g | ➤ Subscribe | 2005-08-23 |
| ➤ Subscribe | Delayed-release Tablets | 75 mg/0.2 mg | ➤ Subscribe | 2008-11-28 |
| ➤ Subscribe | Extended-release Tablets | 4 mg and 8 mg | ➤ Subscribe | 2012-10-31 |
| ➤ Subscribe | Injection | 20 mg/mL, 2 mL and 5 mL vials | ➤ Subscribe | 2004-07-26 |
| ➤ Subscribe | Capsules | 5 mg and 10 mg | ➤ Subscribe | 2005-06-21 |
| ➤ Subscribe | For Injection | 500 mg/vial | ➤ Subscribe | 2011-06-17 |
| ➤ Subscribe | Oral Suspension | 100 mg/5 mL | ➤ Subscribe | 2009-08-03 |
| ➤ Subscribe | Injection | 2 mg/mL, 100 mL bag | ➤ Subscribe | 2009-12-29 |
International Patents for PFIZER Drugs
Supplementary Protection Certificates for PFIZER Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2170860 | 36/2020 | Austria | ⤷ Sign Up | PRODUCT NAME: GLASDEGIB, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEHMBAREN SALZES, EINSCHLIESSLICH DES MALEATSALZES; REGISTRATION NO/DATE: EU/1/20/1451/001 (MITTEILUNG) 20200629 |
| 0454511 | 99C0009 | Belgium | ⤷ Sign Up | PRODUCT NAME: IRBESARTAN / HYDROCHLOROTHIAZIDE; REGISTRATION NO/DATE: EU/1/98/086/001 19981015 |
| 1746999 | SPC/GB19/053 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: DACOMITINIB, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, SUCH AS DACOMITINIB MONOHYDRATE; REGISTERED: UK EU/1/19/1354(FOR NI) 20190404; UK PLGB 00057-1677 20190404; UK PLGB 00057-1678 20190404 |
| 1470124 | 132017000046148 | Italy | ⤷ Sign Up | PRODUCT NAME: PALBOCICLIB, OPZIONALMENTE NELLA FORMA DI UN SALE, ESTERE, AMMIDE O PROFARMACO FARMACEUTICAMENTE ACCETTABILE(IBRANCE); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/16/1147/001-006, 20161111 |
| 2958921 | 2022C/505 | Belgium | ⤷ Sign Up | PRODUCT NAME: ABROCITINIB, OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; AUTHORISATION NUMBER AND DATE: EU/1/21/1593 20211210 |
| 2488512 | CA 2022 00036 | Denmark | ⤷ Sign Up | PRODUCT NAME: RIMEGEPANT ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/22/1645 20220426 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.