IBRANCE Drug Patent Profile
✉ Email this page to a colleague
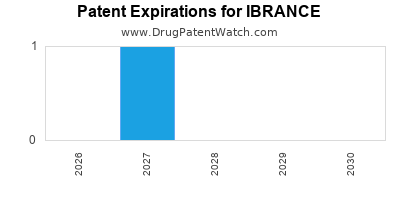
When do Ibrance patents expire, and when can generic versions of Ibrance launch?
Ibrance is a drug marketed by Pfizer and is included in two NDAs. There are three patents protecting this drug and two Paragraph IV challenges.
This drug has one hundred and sixty-nine patent family members in fifty-six countries.
The generic ingredient in IBRANCE is palbociclib. There are thirteen drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the palbociclib profile page.
DrugPatentWatch® Generic Entry Outlook for Ibrance
Ibrance was eligible for patent challenges on February 3, 2019.
There have been thirty-seven patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are six tentative approvals for the generic drug (palbociclib), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for IBRANCE?
- What are the global sales for IBRANCE?
- What is Average Wholesale Price for IBRANCE?
Summary for IBRANCE
| International Patents: | 169 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 128 |
| Patent Applications: | 4,925 |
| Drug Prices: | Drug price information for IBRANCE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for IBRANCE |
| What excipients (inactive ingredients) are in IBRANCE? | IBRANCE excipients list |
| DailyMed Link: | IBRANCE at DailyMed |

Recent Clinical Trials for IBRANCE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University Medical Center Groningen | Phase 4 |
| Megalabs | PHASE4 |
| AstraZeneca | Phase 1/Phase 2 |
Pharmacology for IBRANCE
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Cytochrome P450 3A Inhibitors Kinase Inhibitors |
Paragraph IV (Patent) Challenges for IBRANCE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| IBRANCE | Tablets | palbociclib | 75 mg, 100 mg and 125 mg | 212436 | 1 | 2020-11-24 |
| IBRANCE | Capsules | palbociclib | 75 mg, 100 mg and 125 mg | 207103 | 12 | 2019-02-04 |
US Patents and Regulatory Information for IBRANCE
IBRANCE is protected by three US patents and two FDA Regulatory Exclusivities.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pfizer | IBRANCE | palbociclib | CAPSULE;ORAL | 207103-001 | Feb 3, 2015 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Pfizer | IBRANCE | palbociclib | TABLET;ORAL | 212436-002 | Nov 1, 2019 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Pfizer | IBRANCE | palbociclib | TABLET;ORAL | 212436-003 | Nov 1, 2019 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Pfizer | IBRANCE | palbociclib | CAPSULE;ORAL | 207103-002 | Feb 3, 2015 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Pfizer | IBRANCE | palbociclib | CAPSULE;ORAL | 207103-001 | Feb 3, 2015 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for IBRANCE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Pfizer | IBRANCE | palbociclib | TABLET;ORAL | 212436-001 | Nov 1, 2019 | ⤷ Get Started Free | ⤷ Get Started Free |
| Pfizer | IBRANCE | palbociclib | CAPSULE;ORAL | 207103-002 | Feb 3, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| Pfizer | IBRANCE | palbociclib | CAPSULE;ORAL | 207103-001 | Feb 3, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| Pfizer | IBRANCE | palbociclib | CAPSULE;ORAL | 207103-002 | Feb 3, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| Pfizer | IBRANCE | palbociclib | CAPSULE;ORAL | 207103-003 | Feb 3, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for IBRANCE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Pfizer Europe MA EEIG | Ibrance | palbociclib | EMEA/H/C/003853Ibrance is indicated for the treatment of hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative locally advanced or metastatic breast cancer:in combination with an aromatase inhibitor;in combination with fulvestrant in women who have received prior endocrine therapy.In pre- or perimenopausal women, the endocrine therapy should be combined with a luteinizing hormone releasing hormone (LHRH) agonist. | Authorised | no | no | no | 2016-11-09 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for IBRANCE
When does loss-of-exclusivity occur for IBRANCE?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 4842
Estimated Expiration: ⤷ Get Started Free
Patent: 4909
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 14220354
Estimated Expiration: ⤷ Get Started Free
Patent: 16272881
Estimated Expiration: ⤷ Get Started Free
Patent: 19204689
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2015019508
Estimated Expiration: ⤷ Get Started Free
Patent: 2017025398
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 00322
Estimated Expiration: ⤷ Get Started Free
Patent: 31892
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 17003089
Estimated Expiration: ⤷ Get Started Free
China
Patent: 5008357
Estimated Expiration: ⤷ Get Started Free
Patent: 7666914
Estimated Expiration: ⤷ Get Started Free
Patent: 7759594
Estimated Expiration: ⤷ Get Started Free
Patent: 1253394
Estimated Expiration: ⤷ Get Started Free
Patent: 3616606
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 17012362
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 170540
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0192065
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 20734
Estimated Expiration: ⤷ Get Started Free
Patent: 22454
Estimated Expiration: ⤷ Get Started Free
Patent: 24068
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 58916
Estimated Expiration: ⤷ Get Started Free
Patent: 02565
Estimated Expiration: ⤷ Get Started Free
Patent: 31475
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 017000280
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 17085737
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 58916
Estimated Expiration: ⤷ Get Started Free
Patent: 02565
Estimated Expiration: ⤷ Get Started Free
Patent: 31475
Estimated Expiration: ⤷ Get Started Free
Patent: 36283
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 11032
Estimated Expiration: ⤷ Get Started Free
Patent: 48217
Estimated Expiration: ⤷ Get Started Free
Patent: 50570
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 40434
Estimated Expiration: ⤷ Get Started Free
Patent: 47477
Estimated Expiration: ⤷ Get Started Free
Patent: 54212
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 0277
Estimated Expiration: ⤷ Get Started Free
Patent: 5632
Estimated Expiration: ⤷ Get Started Free
Patent: 7437
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 81016
Estimated Expiration: ⤷ Get Started Free
Patent: 24152
Estimated Expiration: ⤷ Get Started Free
Patent: 27302
Estimated Expiration: ⤷ Get Started Free
Patent: 14162794
Estimated Expiration: ⤷ Get Started Free
Patent: 17002034
Estimated Expiration: ⤷ Get Started Free
Patent: 17186376
Estimated Expiration: ⤷ Get Started Free
Patent: 19116512
Estimated Expiration: ⤷ Get Started Free
Patent: 21167343
Estimated Expiration: ⤷ Get Started Free
Patent: 23112149
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 02565
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 3715
Estimated Expiration: ⤷ Get Started Free
Patent: 6083
Estimated Expiration: ⤷ Get Started Free
Patent: 6473
Estimated Expiration: ⤷ Get Started Free
Patent: 15010858
Estimated Expiration: ⤷ Get Started Free
Patent: 17015579
Estimated Expiration: ⤷ Get Started Free
Patent: 19003605
Estimated Expiration: ⤷ Get Started Free
Patent: 20003825
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 0138
Estimated Expiration: ⤷ Get Started Free
Patent: 7391
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 180395
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 58916
Estimated Expiration: ⤷ Get Started Free
Patent: 02565
Estimated Expiration: ⤷ Get Started Free
Patent: 31475
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 58916
Estimated Expiration: ⤷ Get Started Free
Patent: 02565
Estimated Expiration: ⤷ Get Started Free
Patent: 31475
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 19944
Estimated Expiration: ⤷ Get Started Free
Patent: 86840
Estimated Expiration: ⤷ Get Started Free
Patent: 15132371
Estimated Expiration: ⤷ Get Started Free
Saudi Arabia
Patent: 7390473
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 672
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201505680R
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 58916
Estimated Expiration: ⤷ Get Started Free
Patent: 02565
Estimated Expiration: ⤷ Get Started Free
Patent: 31475
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1707780
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1858913
Estimated Expiration: ⤷ Get Started Free
Patent: 2068423
Estimated Expiration: ⤷ Get Started Free
Patent: 2369405
Estimated Expiration: ⤷ Get Started Free
Patent: 150107872
Estimated Expiration: ⤷ Get Started Free
Patent: 170094012
Estimated Expiration: ⤷ Get Started Free
Patent: 180015232
Estimated Expiration: ⤷ Get Started Free
Patent: 200006633
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 94787
Estimated Expiration: ⤷ Get Started Free
Patent: 64459
Estimated Expiration: ⤷ Get Started Free
Patent: 69277
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 33103
Estimated Expiration: ⤷ Get Started Free
Patent: 35863
Estimated Expiration: ⤷ Get Started Free
Patent: 70269
Estimated Expiration: ⤷ Get Started Free
Patent: 63881
Estimated Expiration: ⤷ Get Started Free
Patent: 1444834
Estimated Expiration: ⤷ Get Started Free
Patent: 1711687
Estimated Expiration: ⤷ Get Started Free
Patent: 1803872
Estimated Expiration: ⤷ Get Started Free
Patent: 1906611
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1816077
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering IBRANCE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Colombia | 5700765 | 2-(PIRIDIN-2-ILAMINO)-PIRIDO[2,3-D] PIRIMIDIN-7-ONAS | ⤷ Get Started Free |
| Japan | 2005519909 | ⤷ Get Started Free | |
| South Korea | 20180015232 | ⤷ Get Started Free | |
| Canada | 2931892 | ⤷ Get Started Free | |
| Portugal | 2958916 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for IBRANCE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1470124 | 300863 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: PALBOCICLIB, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT; REGISTRATION NO/DATE: EU/1/16/1147 20161111 |
| 1470124 | SPC/GB17/026 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: PALBOCICLIB, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, ESTER, AMIDE OR PRODRUG THEREOF; REGISTERED: UK EU/1/16/1147 20161109; UK FURTHER MAS ON IPSUM 20161109 |
| 1470124 | LUC00009 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: PALBOCICLIB, EVENTUELLEMENT SOUS LA FORME D'UN SEL , D'UN ESTER, D'UN AMIDE OU D'UN PROMEDICAMENT PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; AUTHORISATION NUMBER AND DATE: EU/1/16/1147 20161111 |
| 1470124 | C20170012 00212 | Estonia | ⤷ Get Started Free | PRODUCT NAME: PALBOTSIKLIIB;REG NO/DATE: EU/1/16/1147 11.11.2016 |
| 1470124 | C 2017 016 | Romania | ⤷ Get Started Free | PRODUCT NAME: PALBOCICLIB OPTIONAL SUB FORMA DE SARE, ESTER, AMIDA SAUPROMEDICAMENT AL ACESTUIA, ACCEPTABILEFARMACEUTIC; NATIONAL AUTHORISATION NUMBER: EU/1/16/1147; DATE OF NATIONAL AUTHORISATION: 20161109; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/16/1147; DATE OF FIRST AUTHORISATION IN EEA: 20161109 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for IBRANCE
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
