Chiesi Company Profile
✉ Email this page to a colleague
What is the competitive landscape for CHIESI, and when can generic versions of CHIESI drugs launch?
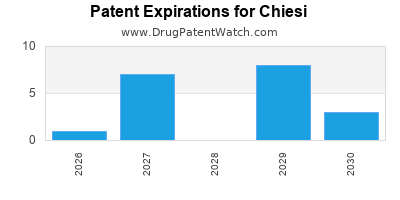
CHIESI has fifteen approved drugs.
There are forty-nine US patents protecting CHIESI drugs.
There are three hundred and seven patent family members on CHIESI drugs in forty-two countries and fifty-three supplementary protection certificates in eighteen countries.
Summary for Chiesi
| International Patents: | 307 |
| US Patents: | 49 |
| Tradenames: | 16 |
| Ingredients: | 10 |
| NDAs: | 15 |
| Patent Litigation for Chiesi: | See patent lawsuits for Chiesi |
Drugs and US Patents for Chiesi
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chiesi | JUXTAPID | lomitapide mesylate | CAPSULE;ORAL | 203858-006 | Apr 23, 2015 | DISCN | Yes | No | 8,618,135 | ⤷ Sign Up | ⤷ Sign Up | ||||
| Chiesi | CARDENE IN 4.8% DEXTROSE IN PLASTIC CONTAINER | nicardipine hydrochloride | INJECTABLE;INTRAVENOUS | 019734-002 | Jul 31, 2008 | RX | Yes | Yes | 7,659,291 | ⤷ Sign Up | ⤷ Sign Up | ||||
| Chiesi | MYCAPSSA | octreotide acetate | CAPSULE, DELAYED RELEASE;ORAL | 208232-001 | Jun 26, 2020 | RX | Yes | Yes | 11,969,471 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Chiesi | CLEVIPREX | clevidipine | EMULSION;INTRAVENOUS | 022156-003 | Nov 8, 2013 | DISCN | Yes | No | 10,010,537 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Chiesi
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Chiesi | ZYFLO | zileuton | TABLET;ORAL | 020471-001 | Dec 9, 1996 | 4,873,259 | ⤷ Sign Up |
| Chiesi | FERRIPROX | deferiprone | TABLET;ORAL | 021825-002 | Jul 25, 2019 | 7,049,328 | ⤷ Sign Up |
| Chiesi | CLEVIPREX | clevidipine | EMULSION;INTRAVENOUS | 022156-003 | Nov 8, 2013 | 5,739,152 | ⤷ Sign Up |
| Chiesi | CARDENE SR | nicardipine hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 020005-003 | Feb 21, 1992 | 5,198,226 | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for CHIESI drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Injection | 0.1 mg/mL, 200 mL and0.2mg/mL, 200 mL | ➤ Subscribe | 2013-06-20 |
| ➤ Subscribe | Tablets | 500 mg | ➤ Subscribe | 2016-01-29 |
| ➤ Subscribe | Injection | 2.5 mg/mL, 10 mL Ampoules | ➤ Subscribe | 2006-12-27 |
| ➤ Subscribe | Inhalation Solution | 300 mg/4 mL | ➤ Subscribe | 2017-08-31 |
International Patents for Chiesi Drugs
Supplementary Protection Certificates for Chiesi Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1725234 | 14C0003 | France | ⤷ Sign Up | PRODUCT NAME: LOMITAPIDE , OU UN SEL PHARMACEUTIQUEMENT ACTIF DE CELUI-CI.; REGISTRATION NO/DATE: EU/1/13/851/001 20130805 |
| 0726894 | C300520 | Netherlands | ⤷ Sign Up | PRODUCT NAME: CLEVIDIPINE; NAT. REGISTRATION NO/DATE: RVG 104771 20111129; FIRST REGISTRATION: PL 16881/0003 20111123 |
| 1725234 | 132014902228079 | Italy | ⤷ Sign Up | PRODUCT NAME: LOMITAPIDE E OGNI SUA FORMA TERAPEUTICAMENTE EQUIVALENTE COME PROTETTA DAL BREVETTO BASE(LOJUXTA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/13/851/001/-003, 20130805 |
| 1725234 | 92349 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: LOMITAPIDE ET TOUTES SES FORMES THERAPEUTIQUEMENT EQUIVALENTES TELLES QUE PROTEGEES PAR LE BREVET DE BASE. FIRST REGISTRATION: 20130805 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.