Last updated: July 27, 2025
Introduction
Bausch, a prominent player in the global ophthalmology and eye health market, has established itself through a focus on innovative ophthalmic products, diagnostic devices, and eye surgery solutions. The company's strategic positioning stems from a rich heritage, a diversified product portfolio, and an emphasis on R&D. As the pharmaceutical landscape evolves amidst technological advances and increasing competition, understanding Bausch's market stance, strengths, and future directions becomes critical for stakeholders aiming to capitalize on ophthalmic treatment opportunities.
Market Position of Bausch
Bausch's footprint in the ophthalmology sector is distinguished by its extensive product lineup covering prescription medications, surgical devices, and diagnostics. The company's strategic emphasis on eye health has yielded a robust global market presence, especially in skincare, contact lens solutions, and surgical products.
According to recent industry reports, Bausch is ranked among the top tier of ophthalmic pharmaceutical companies, competing directly with giants such as Alcon, Johnson & Johnson Vision, and Novartis Vision Care. Its core segments include:
- Prescription ophthalmic drugs: including treatments for glaucoma, dry eye syndrome, and infection.
- Surgical devices: intraocular lenses, vitreoretinal surgery equipment.
- Diagnostic devices: tonometers and imaging systems for eye assessment.
Bausch’s geographic footprint spans North America, Europe, and parts of Asia, with strategic acquisitions bolstering its market share. Notably, the acquisition of Swiss-based corporate entities has enhanced its R&D capabilities and international reach, propelling it into emerging markets with tailored ophthalmic solutions.
Core Strengths of Bausch
1. Focused Ophthalmology Portfolio
Bausch's concentrated focus on ophthalmology differentiates it from diversified pharmaceutical companies. Its specialized portfolio addresses high-prevalence eye conditions, creating consistent demand streams. Its flagship products, such as Lumify for eye pain and Restasis for dry eye disease, underscore its commitment to addressing unmet clinical needs.
2. Robust R&D and Innovation
Investment in R&D is central to Bausch’s strategic advantage. With dedicated research centers and collaborations with academic institutions, Bausch consistently advances new formulations and delivery methods. Its pipeline includes innovative treatments for glaucoma and age-related macular degeneration (AMD), positioning it at the forefront of ophthalmic therapeutics.
3. Strong Distribution and Global Reach
Bausch's extensive distribution network ensures widespread access to its products, complemented by strategic partnerships with healthcare providers, hospitals, and eye clinics worldwide. Its emphasis on expanding in emerging markets leverages unmet medical needs and favorable regulatory environments.
4. Commercial Synergies Through Acquisitions
Recent acquisitions, including Ferrari Pharamaceuticals and other regional entities, have expanded Bausch’s product lines and enhanced market penetration. These strategic moves optimize economies of scale and facilitate the deployment of innovative solutions across diverse geographies.
5. Regulatory Expertise and Clinical Efficacy
Bausch's ability to secure regulatory approvals across multiple jurisdictions facilitates swift product launches and maintains compliance standards. Its emphasis on clinical evidence underpins product credibility and sustains market trust.
Strategic Insights
Market Trends and Opportunities
-
Growing Prevalence of Eye Diseases: Global aging populations are driving increased incidence of glaucoma, AMD, and diabetic retinopathy. Bausch’s portfolio is well-positioned to capitalize on these trends with targeted therapeutics and surgical solutions.
-
Adoption of Minimally Invasive Surgery (MIS): The trend toward less invasive procedures favors products like advanced intraocular lenses and vitreoretinal devices, areas where Bausch has significant expertise.
-
Digital and Diagnostic Innovation: Incorporating AI and telemedicine into eye health diagnostics presents opportunities for Bausch to develop digital health solutions complementing its hardware and pharmaceutical offerings.
-
Emerging Markets Expansion: Countries like India and Brazil exhibit significant growth potential. Bausch’s tailored strategies, including local manufacturing and regulatory collaborations, foster expansion in these regions.
Challenges and Competitive Strategies
-
Intense Competition: Alcon and Johnson & Johnson dominate global market share, necessitating aggressive R&D, strategic partnerships, and differentiated marketing for Bausch.
-
Regulatory Hurdles: Variability in approval processes can delay product launches. Bausch must navigate evolving regulations adeptly through proactive regulatory affairs.
-
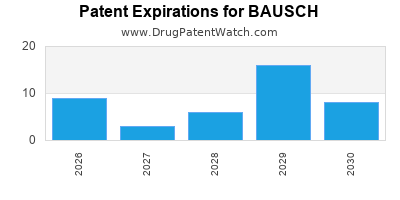
Patent Expirations and Generic Competition: Bausch’s reliance on patent-protected drugs faces the threat of generic entry. Innovating new formulations and securing new patents remain vital strategies.
-
Cost Pressures: Pricing pressures, especially in emerging markets, require cost-effective manufacturing and distribution models.
Future Strategic Directions
-
Pipeline Expansion: Investing in next-generation ophthalmic drugs and devices, particularly those leveraging gene therapy or biologics, aligns with market advancements.
-
Partnerships and Collaborations: Strategic alliances with digital health firms and academic institutions can augment Bausch’s innovation capabilities.
-
Sustainable and Patient-Centric Approaches: Emphasizing patient-friendly devices and eco-friendly manufacturing practices will enhance brand reputation and compliance.
Key Takeaways
- Bausch’s specialized ophthalmology focus, robust R&D, and extensive global distribution underpin its competitive advantage.
- Strategic acquisitions and geographic expansion are critical for growth, especially in emerging markets.
- The company's ability to innovate in minimally invasive surgical technologies and digital diagnostics will be pivotal in capturing future market share.
- Challenges such as intense competition, regulatory variability, and patent cliffs necessitate proactive planning and continuous innovation.
- Long-term success hinges on integrating digital health and personalized medicine into its core offerings.
FAQs
1. How does Bausch differentiate itself from competitors like Alcon and J&J Vision?
Bausch emphasizes a dedicated ophthalmology portfolio with a focus on high-growth segments like dry eye disease and glaucoma, coupled with a strategic emphasis on R&D and regional market expansion, enabling it to offer specialized, innovative solutions with tailored regional strategies.
2. What are the primary growth areas for Bausch in the next five years?
Key growth drivers include expanding into emerging markets, advancing minimally invasive surgical devices, and integrating digital health technologies such as AI-driven diagnostics.
3. How does Bausch address regulatory challenges across different regions?
The company maintains dedicated regulatory affairs teams and collaborations to streamline approval processes, adapt to regional standards, and expedite product launches globally.
4. What impact do patent expirations pose to Bausch?
Patent expirations threaten revenue from flagship drugs; thus, Bausch invests heavily in pipeline development and alternative formulations to mitigate generic competition.
5. How might Bausch leverage digital health innovations?
By integrating AI-powered diagnostics, teleophthalmology services, and data analytics, Bausch can enhance early detection, personalized treatment, and remote patient management, strengthening its market position.
References
[1] Industry reports on the ophthalmic pharmaceutical market.
[2] Bausch’s official annual report and investor presentations.
[3] Market analysis articles from pharmaceutical industry publications.