AMNEAL Company Profile
✉ Email this page to a colleague
What is the competitive landscape for AMNEAL, and when can generic versions of AMNEAL drugs launch?
AMNEAL has three hundred and ninety approved drugs.
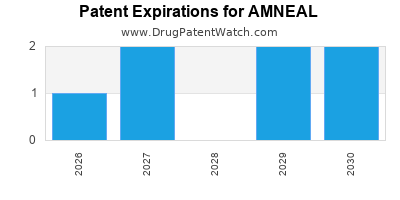
There are fourteen US patents protecting AMNEAL drugs. There are seven tentative approvals on AMNEAL drugs.
There are one hundred and twenty patent family members on AMNEAL drugs in thirty-one countries and eight hundred and twenty-two supplementary protection certificates in nineteen countries.
Summary for AMNEAL
| International Patents: | 120 |
| US Patents: | 14 |
| Tradenames: | 296 |
| Ingredients: | 288 |
| NDAs: | 390 |
| Patent Litigation for AMNEAL: | See patent lawsuits for AMNEAL |
| PTAB Cases with AMNEAL as petitioner: | See PTAB cases with AMNEAL as petitioner |
Drugs and US Patents for AMNEAL
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amneal Pharms | MONTELUKAST SODIUM | montelukast sodium | TABLET;ORAL | 204604-001 | Sep 4, 2015 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Amneal Pharms Co | EZETIMIBE AND SIMVASTATIN | ezetimibe; simvastatin | TABLET;ORAL | 208831-001 | Nov 21, 2017 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Amneal Pharms Ny | ALPRAZOLAM | alprazolam | TABLET, EXTENDED RELEASE;ORAL | 078387-001 | May 30, 2008 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Amneal | VASOPRESSIN | vasopressin | SOLUTION;INTRAVENOUS | 212944-001 | Aug 5, 2022 | AP | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Amneal | BUPRENORPHINE | buprenorphine | FILM, EXTENDED RELEASE;TRANSDERMAL | 211586-002 | Apr 14, 2020 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Amneal Pharms | METHYLPHENIDATE HYDROCHLORIDE | methylphenidate hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 207515-003 | Feb 1, 2018 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for AMNEAL
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Amneal | ACTIVELLA | estradiol; norethindrone acetate | TABLET;ORAL | 020907-001 | Nov 18, 1998 | RE36247 | ⤷ Sign Up |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-003 | Sep 16, 2013 | 6,750,237*PED | ⤷ Sign Up |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-004 | Sep 30, 2003 | 5,466,699*PED | ⤷ Sign Up |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-004 | Sep 30, 2003 | 7,220,767*PED | ⤷ Sign Up |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-004 | Sep 30, 2003 | 6,750,237*PED | ⤷ Sign Up |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-003 | Sep 16, 2013 | 7,220,767*PED | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for AMNEAL drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Nasal Spray | 5 mg/spray | ➤ Subscribe | 2013-11-14 |
| ➤ Subscribe | for Injection | 100 mcg/vial and 500 mcg/vial | ➤ Subscribe | 2015-04-14 |
| ➤ Subscribe | Injection | 100 mg/mL, 2.5 mL vials | ➤ Subscribe | 2007-09-24 |
| ➤ Subscribe | Nasal Spray | 2.5 mg/spray | ➤ Subscribe | 2016-06-09 |
| ➤ Subscribe | for Injection | 200 mcg/vial | ➤ Subscribe | 2015-05-01 |
| ➤ Subscribe | Injection | 1 mg/mL, 50 mL vials | ➤ Subscribe | 2011-12-16 |
International Patents for AMNEAL Drugs
Supplementary Protection Certificates for AMNEAL Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1581193 | SPC/GB12/047 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: DEXAMETHASONE; REGISTERED: UK EU/1/10/638/001 20100727 |
| 1429780 | 132013902137451 | Italy | ⤷ Sign Up | PRODUCT NAME: CIPROFLOXACINA E DESAMETASONE(CILODEX); AUTHORISATION NUMBER(S) AND DATE(S): 48976, 20120810;041182015/M, 20121106 |
| 2666774 | LUC00167 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: RELEBACTAM, EVENTUELLEMENT SOUS FORME DE MONOHYDRATE, IMIPENEME ET CILASTATINE, EVENTUELLEMENT SOUS FORME DE SEL DE SODIUM; AUTHORISATION NUMBER AND DATE: EU/1/19/1420 20200217 |
| 2137537 | 1490038-5 | Sweden | ⤷ Sign Up | PRODUCT NAME: DIMETHYL FUMARATE; REG. NO/DATE: EU/1/13/837 20140203 |
| 2487163 | 2016C/066 | Belgium | ⤷ Sign Up | PRODUCT NAME: COBICISTAT ET ATAZANAVIR; AUTHORISATION NUMBER AND DATE: EU/1/15/1025 20150715 |
| 0316704 | SPC/GB01/015 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: CAPECITABINE AND HYDRATES AND SOLVATES THEREOF; REGISTERED: CH 54657 19980610; UK EU/1/00/163/001-002 20010202 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.