ZIOPTAN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Zioptan, and what generic alternatives are available?
Zioptan is a drug marketed by Thea Pharma and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has eighty patent family members in twenty-six countries.
The generic ingredient in ZIOPTAN is tafluprost. There are three drug master file entries for this compound. Four suppliers are listed for this compound. Additional details are available on the tafluprost profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Zioptan
A generic version of ZIOPTAN was approved as tafluprost by MICRO LABS on August 19th, 2019.
Summary for ZIOPTAN
| International Patents: | 80 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 56 |
| Patent Applications: | 533 |
| Formulation / Manufacturing: | see details |
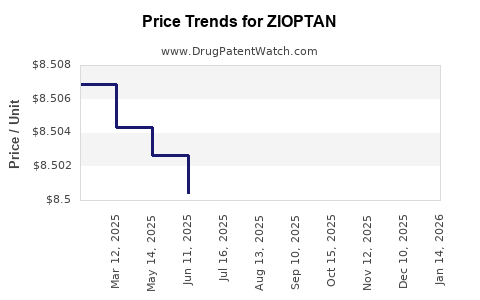
| Drug Prices: | Drug price information for ZIOPTAN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ZIOPTAN |
| What excipients (inactive ingredients) are in ZIOPTAN? | ZIOPTAN excipients list |
| DailyMed Link: | ZIOPTAN at DailyMed |
Pharmacology for ZIOPTAN
| Drug Class | Prostaglandin Analog |
| Mechanism of Action | Prostaglandin Receptor Agonists |
| Physiological Effect | Increased Prostaglandin Activity |
Anatomical Therapeutic Chemical (ATC) Classes for ZIOPTAN
Paragraph IV (Patent) Challenges for ZIOPTAN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ZIOPTAN | Ophthalmic Solution | tafluprost | 0.0015% | 202514 | 2 | 2016-02-10 |
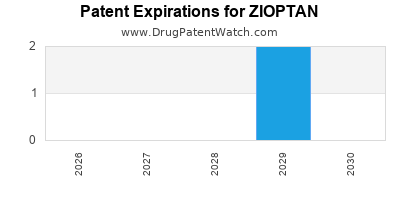
US Patents and Regulatory Information for ZIOPTAN
ZIOPTAN is protected by two US patents.
Patents protecting ZIOPTAN
Method and composition for treating ocular hypertension and glaucoma
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: REDUCTION OF ELEVATED INTRAOCULAR PRESSURE IN PATIENTS WITH OPEN ANGLE GLAUCOMA OR OCULAR HYPERTENSION
Method and composition for treating ocular hypertension and glaucoma
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thea Pharma | ZIOPTAN | tafluprost | SOLUTION/DROPS;OPHTHALMIC | 202514-001 | Feb 10, 2012 | AT | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Thea Pharma | ZIOPTAN | tafluprost | SOLUTION/DROPS;OPHTHALMIC | 202514-001 | Feb 10, 2012 | AT | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ZIOPTAN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Thea Pharma | ZIOPTAN | tafluprost | SOLUTION/DROPS;OPHTHALMIC | 202514-001 | Feb 10, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ZIOPTAN
When does loss-of-exclusivity occur for ZIOPTAN?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 1937
Estimated Expiration: ⤷ Try a Trial
Patent: 0961
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 09252210
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0913109
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 24194
Estimated Expiration: ⤷ Try a Trial
Patent: 65185
Estimated Expiration: ⤷ Try a Trial
China
Patent: 2083413
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0140979
Estimated Expiration: ⤷ Try a Trial
Patent: 0170769
Estimated Expiration: ⤷ Try a Trial
Patent: 0200998
Estimated Expiration: ⤷ Try a Trial
Patent: 0220361
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 15565
Estimated Expiration: ⤷ Try a Trial
Patent: 20351
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 06977
Estimated Expiration: ⤷ Try a Trial
Patent: 72249
Estimated Expiration: ⤷ Try a Trial
Patent: 05334
Estimated Expiration: ⤷ Try a Trial
Patent: 14877
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 3661
Estimated Expiration: ⤷ Try a Trial
Patent: 1071413
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 27638
Estimated Expiration: ⤷ Try a Trial
Patent: 06977
Estimated Expiration: ⤷ Try a Trial
Patent: 72249
Estimated Expiration: ⤷ Try a Trial
Patent: 05334
Estimated Expiration: ⤷ Try a Trial
Patent: 14877
Estimated Expiration: ⤷ Try a Trial
Patent: 35656
Estimated Expiration: ⤷ Try a Trial
Patent: 89446
Estimated Expiration: ⤷ Try a Trial
Georgia, Republic of
Patent: 0156220
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 33103
Estimated Expiration: ⤷ Try a Trial
Patent: 49923
Estimated Expiration: ⤷ Try a Trial
Patent: 58079
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 48317
Estimated Expiration: ⤷ Try a Trial
Patent: 56868
Estimated Expiration: ⤷ Try a Trial
Patent: 49992
Estimated Expiration: ⤷ Try a Trial
Patent: 89789
Estimated Expiration: ⤷ Try a Trial
Patent: 65584
Estimated Expiration: ⤷ Try a Trial
Patent: 11521943
Estimated Expiration: ⤷ Try a Trial
Patent: 14133765
Estimated Expiration: ⤷ Try a Trial
Patent: 16065095
Estimated Expiration: ⤷ Try a Trial
Patent: 17178957
Estimated Expiration: ⤷ Try a Trial
Patent: 18154656
Estimated Expiration: ⤷ Try a Trial
Patent: 20073574
Estimated Expiration: ⤷ Try a Trial
Patent: 21120412
Estimated Expiration: ⤷ Try a Trial
Patent: 23085558
Estimated Expiration: ⤷ Try a Trial
Jordan
Patent: 39
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 72249
Estimated Expiration: ⤷ Try a Trial
Patent: 05334
Estimated Expiration: ⤷ Try a Trial
Patent: 14877
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 9463
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 10012987
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 06977
Estimated Expiration: ⤷ Try a Trial
Patent: 72249
Estimated Expiration: ⤷ Try a Trial
Patent: 05334
Estimated Expiration: ⤷ Try a Trial
Patent: 14877
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 06977
Estimated Expiration: ⤷ Try a Trial
Patent: 72249
Estimated Expiration: ⤷ Try a Trial
Patent: 05334
Estimated Expiration: ⤷ Try a Trial
Patent: 14877
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 1628
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 06977
Estimated Expiration: ⤷ Try a Trial
Patent: 72249
Estimated Expiration: ⤷ Try a Trial
Patent: 05334
Estimated Expiration: ⤷ Try a Trial
Patent: 14877
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1650006
Estimated Expiration: ⤷ Try a Trial
Patent: 1820816
Estimated Expiration: ⤷ Try a Trial
Patent: 1988642
Estimated Expiration: ⤷ Try a Trial
Patent: 2114401
Estimated Expiration: ⤷ Try a Trial
Patent: 2246598
Estimated Expiration: ⤷ Try a Trial
Patent: 110011707
Estimated Expiration: ⤷ Try a Trial
Patent: 160102319
Estimated Expiration: ⤷ Try a Trial
Patent: 180008905
Estimated Expiration: ⤷ Try a Trial
Patent: 190067272
Estimated Expiration: ⤷ Try a Trial
Patent: 200057801
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 95316
Estimated Expiration: ⤷ Try a Trial
Patent: 27837
Estimated Expiration: ⤷ Try a Trial
Patent: 08050
Estimated Expiration: ⤷ Try a Trial
Patent: 07982
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 32202
Estimated Expiration: ⤷ Try a Trial
Patent: 1000104
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 2257
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ZIOPTAN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 102114401 | ⤷ Try a Trial | |
| Japan | 2011521943 | ⤷ Try a Trial | |
| South Korea | 20200057801 | 고안압증과 녹내장의 치료 방법 및 조성물 (METHOD AND COMPOSITION FOR TREATING OCULAR HYPERTENSION AND GLAUCOMA) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ZIOPTAN
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0850926 | CA 2008 00041 | Denmark | ⤷ Try a Trial | |
| 0850926 | 339 | Finland | ⤷ Try a Trial | |
| 0850926 | 11C0020 | France | ⤷ Try a Trial | PRODUCT NAME: TAFLUPROST; REGISTRATION NO/DATE IN FRANCE: CIS:6 000 728 O DU 20110328; REGISTRATION NO/DATE AT EEC: 43230 DU 20080430 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


