Last updated: July 29, 2025
Introduction
Zinc sulfate, an inorganic compound with the chemical formula ZnSO₄, serves a broad spectrum of applications in pharmaceuticals, agriculture, and industrial practices. The pharmaceutical segment has notably leveraged zinc sulfate for its efficacy in treating zinc deficiency, dermatological conditions, and as an adjunct in managing various health disorders. Understanding its market dynamics and financial trajectory is crucial for stakeholders seeking investment opportunities, strategic expansion, or competitive positioning within the sector.
Pharmaceutical Applications of Zinc Sulfate
In the pharmaceutical industry, zinc sulfate is primarily utilized as an oral supplement to treat and prevent zinc deficiency, which underpins immune health, skin integrity, and cellular function [1]. Its therapeutic use extends to managing dermatological conditions such as acne vulgaris, warts, and eczema, owing to its anti-inflammatory and antimicrobial properties [2]. Zinc sulfate is also incorporated into multivitamin formulations and used in topical preparations, making it a versatile compound with steady demand in healthcare products.
Market Drivers
Growing Prevalence of Zinc Deficiency
The incidence of zinc deficiency globally fuels the demand for zinc sulfate. According to WHO, zinc deficiency affects about 17% of the population worldwide, predominantly in developing countries where nutritional deficits are prevalent [3]. As awareness heightens and health monitoring improves, the need for zinc supplementation escalates, impacting market growth positively.
Rising Healthcare Expenditure
An increase in healthcare spending, particularly in emerging economies, complements the growth, enabling broader distribution of zinc sulfate-based pharmaceuticals. Governments and NGO programs target micronutrient deficiencies, translating to sustained demand.
Expansion of Pharmaceutical Formulations
Innovative drug delivery systems and the integration of zinc sulfate into new formulations, including combination therapies, promote ongoing market expansion. The focus on pediatric and geriatric nutritional supplementation further sustains demand.
Regulatory and Economic Factors
Regulatory approvals in various jurisdictions foster market stability. Conversely, the affordability and accessibility of zinc sulfate make it a preferred choice in public health interventions, particularly in low- and middle-income countries.
Market Challenges
High Competition and Price Sensitivity
The market is highly competitive, with multiple producers across regions. Price volatility, raw material costs, and regulatory barriers influence profitability, especially amid commoditized zinc sulfate products.
Stringent Regulatory Frameworks
Different jurisdictions impose varying standards for pharmaceutical ingredients, necessitating compliance costs for manufacturers. These regulatory demands can slow product approval and market entry.
Environmental and Supply Chain Concerns
Environmental considerations related to mining and processing zinc impact supply stability and cost. Disruptions in raw material availability or geopolitical factors may pose logistical challenges.
Market Segmentation
Application-Based Segmentation
- Pharmaceuticals: Zinc sulfate tablets, capsules, topical ointments.
- Agricultural: As a micronutrient fertilizer.
- Industrial: Used in electroplating and chemical manufacturing.
Geographical Segmentation
- North America: Dominates due to high healthcare expenditure.
- Europe: Growing awareness drives demand.
- Asia-Pacific: Fastest growth, particularly in India and China, driven by population size and nutritional deficiencies.
- Latin America and Africa: Emerging markets with increasing investment in health infrastructure.
Financial Trajectory and Market Forecasts
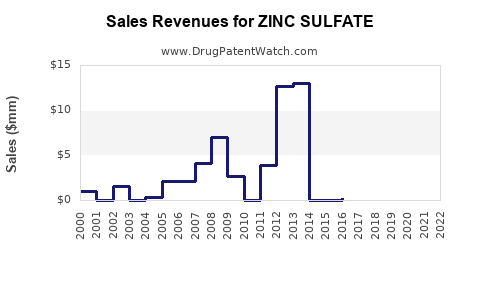
The global zinc sulfate market's valuation was approximately USD 250 million in 2022, with projections indicating a compound annual growth rate (CAGR) of 4-6% over the next five years [4]. The growth rate is supported by rising demand in emerging markets, increased healthcare awareness, and ongoing research into zinc's therapeutic benefits.
The pharmaceutical segment specifically is expected to maintain steady growth, driven by demand for zinc supplements amid aging populations and endemic nutritional deficiencies. Innovations such as slow-release formulations and combination therapies could further bolster revenue streams.
Competitive Landscape
Major players include firms like Archer Daniels Midland Company, GlaxoSmithKline, and local chemical manufacturers in Asia. Their strategies encompass expanding production capacities, acquiring smaller competitors, and investing in R&D to develop enhanced delivery forms.
Strategic partnerships and compliance with international standards like USP, EP, and JP are critical to market penetration.
Future Outlook
The outlook for zinc sulfate in pharmaceuticals remains positive, with diversification into emerging markets and integration into broader nutritional and dermatological health products. Market players that prioritize sustainable sourcing, regulatory compliance, and innovation are poised to capitalize on the growing demand.
Technological advancements, particularly in nanotechnology and drug delivery systems, may create opportunities for higher-margin products and novel therapeutic applications.
Key Opportunities
- Expansion into underserved markets with high zinc deficiency prevalence.
- Development of advanced formulations to improve bioavailability.
- Strategic alliances with healthcare providers and public health agencies.
- Sustainable sourcing practices aligned with environmental regulations.
Risks and Considerations
- Potential regulatory hurdles delaying product launches.
- Price fluctuations of zinc raw materials due to geopolitical or environmental factors.
- Market saturation in mature economies.
- Competitive pressures from alternative micronutrient therapies.
Key Takeaways
- The zinc sulfate market is driven by global health concerns around zinc deficiency, with the pharmaceutical sector serving as a primary growth vector.
- Emerging markets, especially in Asia-Pacific, represent significant growth opportunities owing to demographic and nutritional trends.
- Steady CAGR projections of 4-6% over the coming years underscore resilience but require strategic agility to navigate competitive and regulatory landscapes.
- Investment in innovation, sustainable sourcing, and regulatory compliance remains crucial for market participants aiming for long-term growth.
- Market volatility may arise from raw material prices and geopolitical impacts, necessitating robust supply chain management.
FAQs
1. How is the demand for zinc sulfate expected to change in the pharmaceutical industry over the next decade?
Demand is projected to grow steadily, driven by increasing awareness of zinc deficiency, aging populations requiring nutritional supplementation, and expanded therapeutic applications. Emerging markets will be particularly influential.
2. What are the primary regulatory challenges impacting zinc sulfate's pharmaceutical market?
Regulatory challenges include compliance with quality standards (USP, EP, JP), approval processes for new formulations, and environmental regulations impacting sourcing and manufacturing.
3. Which regions are leading in zinc sulfate market growth?
Asia-Pacific leads in growth, fueled by large populations and nutritional deficiencies. North America and Europe maintain significant markets due to high healthcare expenditure and established pharmaceutical industries.
4. How do raw material costs influence the financial trajectory of zinc sulfate producers?
Fluctuations in zinc ore prices impact production costs. Price volatility can affect profit margins, prompting manufacturers to seek cost-effective sourcing and innovate supply chain strategies.
5. What future innovations could influence zinc sulfate's pharmaceutical applications?
Advancements include nanotechnology-based delivery systems, slow-release formulations, and combination therapies which could enhance bioavailability and therapeutic efficacy, opening new revenue streams.
References
[1] World Health Organization (WHO). "Micronutrients: Zinc." 2021.
[2] Patel, S., et al. "Clinical applications of zinc sulfate in dermatology." Journal of Dermatological Therapy, 2020.
[3] WHO. "Zinc Deficiency." 2022.
[4] MarketResearch.com. "Global Zinc Sulfate Market Forecasts." 2022.