ZELBORAF Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Zelboraf, and when can generic versions of Zelboraf launch?
Zelboraf is a drug marketed by Hoffmann La Roche and is included in one NDA. There are six patents protecting this drug.
This drug has one hundred and ninety-five patent family members in forty-six countries.
The generic ingredient in ZELBORAF is vemurafenib. One supplier is listed for this compound. Additional details are available on the vemurafenib profile page.
DrugPatentWatch® Generic Entry Outlook for Zelboraf
Zelboraf was eligible for patent challenges on August 17, 2015.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 6, 2032. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ZELBORAF?
- What are the global sales for ZELBORAF?
- What is Average Wholesale Price for ZELBORAF?
Summary for ZELBORAF
| International Patents: | 195 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 96 |
| Clinical Trials: | 53 |
| Patent Applications: | 1,764 |
| Drug Prices: | Drug price information for ZELBORAF |
| What excipients (inactive ingredients) are in ZELBORAF? | ZELBORAF excipients list |
| DailyMed Link: | ZELBORAF at DailyMed |
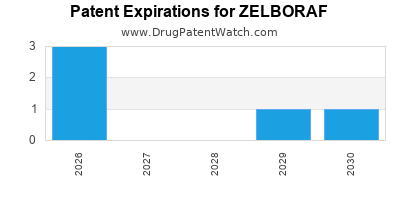

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ZELBORAF
Generic Entry Date for ZELBORAF*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ZELBORAF
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Hoffmann-La Roche | Phase 2/Phase 3 |
| University of Birmingham | Phase 2/Phase 3 |
| Cancer Research UK | Phase 2/Phase 3 |
Pharmacology for ZELBORAF
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Cytochrome P450 1A2 Inhibitors P-Glycoprotein Inhibitors Protein Kinase Inhibitors |
US Patents and Regulatory Information for ZELBORAF
ZELBORAF is protected by seven US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ZELBORAF is ⤷ Get Started Free.
This potential generic entry date is based on patent 9,447,089.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hoffmann La Roche | ZELBORAF | vemurafenib | TABLET;ORAL | 202429-001 | Aug 17, 2011 | RX | Yes | Yes | 8,470,818 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Hoffmann La Roche | ZELBORAF | vemurafenib | TABLET;ORAL | 202429-001 | Aug 17, 2011 | RX | Yes | Yes | 7,504,509 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Hoffmann La Roche | ZELBORAF | vemurafenib | TABLET;ORAL | 202429-001 | Aug 17, 2011 | RX | Yes | Yes | 9,447,089 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Hoffmann La Roche | ZELBORAF | vemurafenib | TABLET;ORAL | 202429-001 | Aug 17, 2011 | RX | Yes | Yes | 8,143,271 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Hoffmann La Roche | ZELBORAF | vemurafenib | TABLET;ORAL | 202429-001 | Aug 17, 2011 | RX | Yes | Yes | 8,741,920 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Hoffmann La Roche | ZELBORAF | vemurafenib | TABLET;ORAL | 202429-001 | Aug 17, 2011 | RX | Yes | Yes | 7,863,288 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ZELBORAF
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Roche Registration GmbH | Zelboraf | vemurafenib | EMEA/H/C/002409Vemurafenib is indicated in monotherapy for the treatment of adult patients with BRAF-V600-mutation-positive unresectable or metastatic melanoma., | Authorised | no | no | no | 2012-02-17 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ZELBORAF
When does loss-of-exclusivity occur for ZELBORAF?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 8033
Patent: UNA DISPERSION SOLIDA, QUE CONTIENE AL COMPUESTO {3-[5-(4-(CLORO-FENIL)-1H-PIRROLO[2,3-B]PIRIDINA-3-CARBONIL]-2,4-DIFLUOR-FENIL}-AMIDA DEL ACIDO PROPANO-1-SULFONICO, COMPOSICIONES Y FORMULACIONES QUE COMPRENDEN A DICHA DISPERSION SOLIDA; METODOS PARA FABRICAR DICHA DISPERSION SOLIDA, FORMAS 1 Y 2 DE
Estimated Expiration: ⤷ Get Started Free
Patent: 1037
Patent: COMPOSICIONES Y USOS DE LAS MISMAS
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 10232670
Patent: Propane- I-sulfonic acid {3- [5- (4 -chloro-phenyl) -1H-pyrrolo [2, 3-b] pyridine-3-carbonyl] -2, 4-difluoro-pheny l } -amide compositions and uses thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 10318049
Patent: Propane-I-sulfonic acid {3-[5-(4-chloro-phenyl)-1H-pyrrolo[2,3-B]pyridine-3-carbonyl]-2,4-difluoro-phenyl}-amide compositions and uses thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 15238857
Patent: Propane- I-sulfonic acid {3- [5- (4 -chloro-phenyl) -1H-pyrrolo [2, 3-b] pyridine-3-carbonyl] -2, 4-difluoro-pheny l } -amide compositions and uses thereof
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 1008709
Patent: composições e usos das mesmas.
Estimated Expiration: ⤷ Get Started Free
Patent: 2012009609
Patent: método para fabricar uma dispersão sólida, dispersão sólida , preparação farmacêutica e composições
Estimated Expiration: ⤷ Get Started Free
Patent: 2020005420
Patent: forma purificada 1 do polimorfo cristalino do composto i
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 38573
Patent: COMPOSITIONS ET UTILISATIONS ASSOCIEES (COMPOSITIONS AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 78693
Patent: COMPOSITIONS DE {3-[5-(4-CHLOROPHENYL)-1H-PYRROLO(2,3-B)PYRIDINE-3-CARBONYL]-2,4-DIFLUOROPHENYL)}-AMIDE DE L'ACIDE PROPANE-1-SULFONIQUE ET LEURS UTILISATIONS (PROPANE-I-SULFONIC ACID {3-[5-(4-CHLORO-PHENYL)-1H-PYRROLO[2,3-B]PYRIDINE-3-CARBONYL]-2,4-DIFLUORO-PHENYL}-AMIDE COMPOSITIONS AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2361870
Patent: Propane- i-sulfonic acid {3- [5- (4 -chloro-phenyl) -1h-pyrrolo [2, 3-b] pyridine-3-carbonyl] -2, 4-difluoro-pheny l } -amide compositions and uses thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 2596953
Patent: Propane-i-sulfonic acid {3-[5-(4-chloro-phenyl)-1h-pyrrolo[2,3-b]pyridine-3-carbonyl]-2,4-difluoro-phenyl}-amide compositions and uses thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 5237530
Patent: Propane-1-sulfonic acid {3-[5-(4-chloro-phenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl]-2,4-difluoro-phenyl}-amide compositions and uses thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 0269838
Patent: 丙烷-1-磺酸{3-[5-(4-氯-苯基)-1H-吡咯并[2,3-b]吡啶-3-羰基]-2,4-二氟-苯基}-酰胺组合物及其用途 (Propane- I-sulfonic acid {3- [5- (4 -chloro-phenyl) -1H-pyrrolo [2, 3-b] pyridine-3-carbonyl] -2, 4-difluoro-pheny l } -amide compositions and uses thereof)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 10296
Patent: COMPOSICIONES DEL ÁCIDO PROPANO-1-SULFÓNICO {3-[5-(4-CLORO-FENIL)-1H-PIRROLO [2,3-B]-PIRIDINA-3-CARBONIL]-2,4-DIFLUORO-FENIL}-AMIDA Y EL USO DE LAS MISMAS
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 110420
Patent: COMPOSICIONES DEL ACIDO PROPANO-1-SULFONICO {3-[5-(4-CLORO-FENIL) -1H-PIRROLO [2,3-B] PIRIDINA-3-CARBONIL] -2,4-DIFLUORO-FENIL}-AMIDA Y EL USO DE LAS MISMAS
Estimated Expiration: ⤷ Get Started Free
Patent: 170089
Patent: COMPOSICIONES DEL ACIDO PROPANO-1--SULFONICO {3-[5-(4-CLORO-FENIL)-1H-PIRROLO [2,3-B] PIRIDINA-3-CARBONIL] -2,4-DIFLUORO-FENIL}-AMIDA Y EL USO DE LAS MISMAS
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0151156
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 16983
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 14356
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 011000291
Patent: COMPOSICIONES DEL ACIDO PROPANO-1-SULFONICO{3-[5-(4-CLORO-FENIL)-1H-PIRROLO [2,3-B]-PIRIDINA-3-CARBONIL]-2,4-DIFLUORO-FENIL}-AMIDA Y EL USO DE LAS MISMAS
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 11011282
Patent: COMPOSICIONES DEL ÁCIDO PROPANO-1-SULFÓNICO {3-[5-(4-CLORO-FENIL)-1H-PIRROLO [2,3-b]-PIRIDINA-3-CARBONIL]-2,4-DIFLUORO-FENIL}-AMIDA Y EL USO DE LAS MISMAS
Estimated Expiration: ⤷ Get Started Free
El Salvador
Patent: 11004004
Patent: COMPOSICIONES DEL ACIDO PROPANO-1-SULFONICO {3-[5-(4-CLORO-FENIL)-1H-PIRROLO [2,3-B]-PIRIDINA-3-CARBONIL]-2,4-DIFLUORO-FENIL}-AMIDA Y EL USO DE LAS MISMAS
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 2924
Patent: ТВЁРДАЯ ФОРМА {3-[5-(4-ХЛОРФЕНИЛ)-1Н-ПИРРОЛО[2,3-b]ПИРИДИН-3-КАРБОНИЛ]-2,4-ДИФТОРФЕНИЛ}АМИДА ПРОПАН-1-СУЛЬФОНОВОЙ КИСЛОТЫ И ЕЁ ПРИМЕНЕНИЕ (SOLID FORM OF PROPANE-1-SULFONIC ACID {3-[5-(4-CHLOROPHENYL)-1H-PYRROLO[2,3-b]PYRIDINE-3-CARBONYL]-2,4-DIFLUOROPHENYL}AMIDE AND USE THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 1116
Patent: КРИСТАЛЛИЧЕСКИЕ ПОЛИМОРФНЫЕ ФОРМЫ {3-[5-(4-ХЛОРФЕНИЛ)-1H-ПИРРОЛО[2,3-b]ПИРИДИН-3-КАРБОНИЛ]-2,4-ДИФТОРФЕНИЛ}АМИДА ПРОПАН-1-СУЛЬФОНОВОЙ КИСЛОТЫ (PROPANE-1-SULFONIC ACID {3-[5-(4-CHLORO-PHENYL)-1H-PYRROLO[2,3-b]PYRIDINE-3-CARBONYL]-2,4-DIFLUORO-PHENYL}AMIDE CRYSTALLINE POLYMORPH FORMS)
Estimated Expiration: ⤷ Get Started Free
Patent: 1190098
Patent: КОМПОЗИЦИИ И ИХ ПРИМЕНЕНИЕ
Estimated Expiration: ⤷ Get Started Free
Patent: 1591240
Patent: КОМПОЗИЦИИ {3-[5-(4-ХЛОРФЕНИЛ)-1H-ПИРРОЛО[2,3-B]ПИРИДИН-3-КАРБОНИЛ]-2,4-ДИФТОРФЕНИЛ}АМИДА ПРОПАН-1-СУЛЬФОНОВОЙ КИСЛОТЫ И ИХ ПРИМЕНЕНИЕ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 14356
Patent: COMPOSITIONS DE {3-[5-(4-CHLOROPHÉNYL)-1H-PYRROLO(2,3-B)PYRIDINE-3-CARBONYL]-2,4-DIFLUOROPHÉNYL)}-AMIDE DE L'ACIDE PROPANE-1-SULFONIQUE ET LEURS UTILISATIONS (PROPANE-I-SULFONIC ACID {3-[5-(4-CHLORO-PHENYL)-1H-PYRROLO[2,3-B]PYRIDINE-3-CARBONYL]-2,4-DIFLUORO-PHENYL}-AMIDE COMPOSITIONS AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 99138
Patent: COMPOSITIONS DE {3-[5-(4-CHLOROPHÉNYL)-1H-PYRROLO(2,3-B)PYRIDINE-3-CARBONYL]-2,4-DIFLUOROPHÉNYL)}-AMIDE DE L'ACIDE PROPANE-1-SULFONIQUE ET LEURS UTILISATIONS (PROPANE-I-SULFONIC ACID {3-[5-(4-CHLORO-PHENYL)-1H-PYRROLO[2,3-B]PYRIDINE-3-CARBONYL]-2,4-DIFLUORO-PHENYL}-AMIDE COMPOSITIONS AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 55180
Patent: COMPOSITIONS D'ACIDE PROPANE-I-SULFONIQUE {3- [5- (4- CHLORO-PHÉNYLE) -1H-PYRROLO [2,3-B} PYRIDINE-3-CARBONYLE] -2,4-DIFLUORO-PHÉNYLE} -AMIDE ET LEURS UTILISATIONS (PROPANE-I-SULFONIC ACID {3- [5- (4- CHLORO-PHENYL) -1H-PYRROLO [2, 3-B} PYRIDINE-3-CARBONYL] -2, 4-DIFLUORO-PHENYL} -AMIDE COMPOSITIONS AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Honduras
Patent: 11002147
Patent: COMPOSICIONES DEL ACIDO PROPANO -1-SULFONICO{3-[5-(4-CLORO-FENIL)-11H-PIRROLO[2,3-B]-PIRIDINA-3-CARBONIL]-2,4 DIFLUORO-FENIL]-AMIDA Y EL USO DE LAS MISMAS
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 68590
Patent: } - 丙烷- -磺酸{ -氯-苯基 -吡咯並 吡啶- -羰基 -二氟-苯基}-醯胺組合物及其用途 (PROPANE- I-SULFONIC ACID 3- [5- (4 -CHLORO-PHENYL) -1H-PYRROLO [2, 3-B] PYRIDINE-3-CARBONYL] -2, 4-DIFLUORO-PHENYL -AMIDE COMPOSITIONS AND USES THEREOF -1-3-[5-(4--)-1H-[23-B]-3-]-24---)
Estimated Expiration: ⤷ Get Started Free
Patent: 17195
Patent: } - 丙烷- -磺酸{ -氯-苯基 -吡咯並 吡啶- -羰基 -二氟-苯基}-醯胺組合物及其用途 (PROPANE I SULFONIC ACID 3 [5 (4 CHLORO PHENYL) 1H PYRROLO [2, 3 B PYRIDINE 3 CARBONYL] 2, 4 DIFLUORO PHENYL AMIDE COMPOSITIONS AND USES THEREOF 1 3 [5 (4 ) 1H [23 B] 3 ] 24)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 27598
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 4328
Patent: דיספרסיה מוצקה של פרופאן-1-חומצה סולפונית {3-[5-(4-כלורו-פניל)-1h-פירולו[3,2-b] פירידין-3-קרבוניל]-4,2-דיפלואורו-פניל}-אמיד תכשיר המכיל אותה ושיטה להכנתה (Solid dispersion comprising propane-1-sulfonic acid {3-[5-(4-chloro-phenyl)-1h-pyrrolo [2,3-b] pyridine-3-carbonyl]-2,4-difluoro-phenyl}-amide, composition comprising the same and method for making the solid dispersion)
Estimated Expiration: ⤷ Get Started Free
Patent: 1336
Patent: תכשירי פרופאן-1-חומצה סולפונית {3-[5-(4-כלורו-פניל)-h1-פירולו[b-3,2] פירידין-3-קרבוניל]-4,2-דיפלואורו-פניל}-אמיד ושימושים בהם (Propane-1-sulfonic acid {3- [5- (4 -chloro-phenyl) -1h-pyrrolo [2, 3-b] pyridine-3-carbonyl] -2, 4-difluoro-phenyl } -amide compositions and uses thereof)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 11942
Estimated Expiration: ⤷ Get Started Free
Patent: 12522791
Estimated Expiration: ⤷ Get Started Free
Patent: 13510813
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 56
Patent: تركيبات { 3-[5-(4-كلورو-فينيل )-أ1-بيرلو [2, 3-ب] بيريدين-3-كربونيل ] -2, 4- ثاني فلورو-فينيل } -أميد بروبان-1-حمض سلفونيك واستخداماتها (PROPANE-1-SULFONIC ACID { 3-[5-(4-CHLORO-PHENYL)-1H-PYRROLO [2,3-B] PYRIDINE -3-CARBONYL]-2,4-DIFLUORO-PHENYL} AMIDE COMPOSITION AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 0737
Patent: PROPANE-I-SULFONIC ACID {3- [5-(4-CHLORO-PHENYL) -1H -PYRROLO [2, 3-B] PYRIDINE-3-CARBONYL] -2,4 DIFLUORO-PHENYL} - AMIDE COMPOSITIONS AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
Patent: 2424
Patent: PROPANE- I-SULFONIC ACID {3- (4-CHLORO-PHENYL)-1H-PYRROLO [2, 3-B] PYRIDINE-3-CARCONYL] -2, 4-DIFLUORO-PHENYL} -AMIDE COMPOSITIONS AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 9923
Patent: COMPOSICIONES DEL ÁCIDO PROPANO-1-SULFÓNICO {3-[5-(4-CLORO-FENIL)- 1H-PIRROLO [2,3-B]-PIRIDINA-3-CARBONIL]-2,4-DIFLUORO-FENIL]-AMIDA Y EL USO DE LAS MISMAS. (PROPANE- I-SULFONIC ACID {3- [5- (4 -CHLORO-PHENYL) -1H-PYRROLO [2, 3-B] PYRIDINE-3-CARBONYL] -2, 4-DIFLUORO-PHENY L } -AMIDE COMPOSITIONS AND USES THEREOF.)
Estimated Expiration: ⤷ Get Started Free
Patent: 11008303
Patent: COMPOSICIONES DEL ACIDO PROPANO-1-SULFONICO {3-[5-(4-CLORO-FENIL)- 1H-PIRROLO [2,3-B]-PIRIDINA-3-CARBONIL]-2,4-DIFLUORO-FENIL}-AMIDA Y EL USO DE LAS MISMAS. (PROPANE- I-SULFONIC ACID {3- [5- (4 -CHLORO-PHENYL) -1H-PYRROLO [2, 3-B] PYRIDINE-3-CARBONYL] -2, 4-DIFLUORO-PHENY L } -AMIDE COMPOSITIONS AND USES THEREOF.)
Estimated Expiration: ⤷ Get Started Free
Patent: 12005224
Patent: NUEVO PROCESO PARA LA MANUFACTURA DE PREPARACIONES FARMACEUTICAS. (PROPANE-I-SULFONIC ACID {3-[5-(4-CHLORO-PHENYL)-1H-PYRROLO[2,3-B] PYRIDINE-3-CARBONYL]-2,4-DIFLUORO-PHENYL}-AMIDE COMPOSITIONS AND USES THEREOF.)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 028
Patent: تراكيب واستخدامات مرتبطة بها
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 4398
Patent: Propane-1-sulfonic acid (3-[5-(4-chloro-phenyl)-1h-pyrrol [2, 3-b] pyridine-3-carbonyl]-2,4-difluoro-phenyl} -amide compositions and uses thereof
Estimated Expiration: ⤷ Get Started Free
Nicaragua
Patent: 1100161
Patent: COMPOSICIONES DEL ÁCIDO PROPANO - 1 - SULFÓNICO { 3 - [5 - (4 - CLORO - FENIL) - 1H - PIRROLO [2, 3-b] - PIRIDINA - 3 - CARBONIL] - 2, 4 - DIFLUORO - FENIL} - AMIDA Y EL USO DE LAS MISMAS
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 120876
Patent: COMPOSICIONES DEL ACIDO PROPANO-1-SULFONICO{3-[5-(4-CLORO-FENIL)-1H-PIRROLO[2,3-B]-PIRIDINA-3-CARBONIL]-2,4-DIFLUORO-FENIL}-AMIDA
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 14356
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 14356
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 12123958
Patent: КОМПОЗИЦИИ {3-[5-(4-ХЛОРФЕНИЛ)-1Н-ПИРРОЛО[2, 3]ПИРИДИН-3-КАРБОНИЛ]-2,4-ДИФТОРФЕНИЛ}АМИДА ПРОПАН-1-СУЛЬФОНОВОЙ КИСЛОТЫ И ИХ ПРИМЕНЕНИЕ
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01500302
Patent: COMPOSIZIONI DI {3-[5-(4-CLORO-FENIL)-1H-PIRROLO[2,3-B]PIRIDIN-3-CARBONIL]-2,4-DIFLUORO-FENIL}-AMMIDE DELL'ACIDO PROPAN-1-SOLFONICO E LORO USI
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 3178
Patent: PROPANE- I-SULFONIC ACID {3- [5- (4 -CHLORO-PHENYL) -1H-PYRROLO [2, 3-B] PYRIDINE-3-CARBONYL] -2, 4-DIFLUORO-PHENY L } -AMIDE COMPOSITIONS AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 14356
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1202937
Patent: PROPANE-I-SULFONIC ACID {3-[5-(4-CHLORO-PHENYL)-1H-PYRROLO[2,3-B]PYRIDINE-3-CARBONYL]-2,4-DIFLUORO-PHENYL}-AMIDE COMPOSITIONS AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1739994
Estimated Expiration: ⤷ Get Started Free
Patent: 120006006
Patent: PROPANE-1-SULFONIC ACID {3-[5-(4-CHLORO-PHENYL)-1H-PYRROLO[2,3-B]PYRIDINE-3-CARBONYL]-2,4-DIFLUORO-PHENYL}-AMIDE COMPOSITIONS AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
Patent: 120101439
Patent: PROPANE-1-SULFONIC ACID {3-[5-(4-CHLORO-PHENYL)-1H-PYRROLO[2,3-B]PYRIDINE-3-CARBONYL]-2,4-DIFLUORO-PHENYL}-AMIDE COMPOSITIONS AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
Patent: 170058465
Patent: 프로판-1-술폰산 {3-[5-(4-클로로-페닐)-1H-피롤로[2,3-b]피리딘-3-카르보닐]-2,4-디플루오로-페닐}-아미드 조성물 및 그의 용도 (-- ------------- PROPANE-1-SULFONIC ACID 3-5-4-CHLORO-PHENYL-1H-PYRROLO23-BPYRIDINE-3-CARBONYL-24-DIFLUORO-PHENYL-AMIDE COMPOSITIONS AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 52386
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 04719
Estimated Expiration: ⤷ Get Started Free
Patent: 1040179
Patent: Compositions and uses therof
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 11000436
Patent: PROPANE- I-SULFONIC ACID {3- [5- (4 -CHLORO-PHENYL) -1H-PYRROLO [2, 3-B] PYRIDINE-3-CARBONYL] -2, 4-DIFLUORO-PHENY L } -AMIDE COMPOSITIONS AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 8842
Patent: ТВЕРДА ДИСПЕРСІЯ, СПОСІБ ЇЇ ОДЕРЖАННЯ, А ТАКОЖ КОМПОЗИЦІЯ І ЛІКАРСЬКА ФОРМА, ЩО ЇЇ МІСТЯТЬ
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 540
Patent: COMPOSICIONES QUE INCLUYEN COMPUESTOS QUE CONTIENEN LA {3-[5-(4-CLORO-FENIL)-1H-PIRROLO[2,3-B]PIRIDINA-3-CARBONIL]-2,4-DIFLUOR-FENIL}-AMIDA DEL ÁCIDO PROPANO-1-SULFÓNICO Y MÉTODOS PARA FABRICAR ESTAS COMPOSICIONES
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ZELBORAF around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| China | 102361870 | Propane- i-sulfonic acid {3- [5- (4 -chloro-phenyl) -1h-pyrrolo [2, 3-b] pyridine-3-carbonyl] -2, 4-difluoro-pheny l } -amide compositions and uses thereof | ⤷ Get Started Free |
| World Intellectual Property Organization (WIPO) | 2005062795 | ⤷ Get Started Free | |
| Argentina | 121037 | COMPOSICIONES Y USOS DE LAS MISMAS | ⤷ Get Started Free |
| Japan | 5511942 | ⤷ Get Started Free | |
| Slovenia | 1696920 | ⤷ Get Started Free | |
| Costa Rica | 9677 | DERIVADOS DE PIRROLO(2,3-B) PIRIDINA COMO INHIBIDORES DE PROTEINA CINASA | ⤷ Get Started Free |
| Poland | 2395004 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ZELBORAF
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1893612 | C 2012 020 | Romania | ⤷ Get Started Free | PRODUCT NAME: VEMURAFENIB SAU O SARE ACCEPTABILA FARMACEUTIC A ACESTUIA; NATIONAL AUTHORISATION NUMBER: EU/1/17/1212; DATE OF NATIONAL AUTHORISATION: 20120217; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/17/1212; DATE OF FIRST AUTHORISATION IN EEA: 20120217 |
| 1893612 | 132012902073472 | Italy | ⤷ Get Started Free | PRODUCT NAME: VEMURAFENIB(ZELBORAF); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/12/751/001, 20120221 |
| 1893612 | 2012/025 | Ireland | ⤷ Get Started Free | PRODUCT NAME: VEMURAFENIB AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTRATION NO/DATE: EU/1/12/751/001 20120217 |
| 1893612 | 1290026-2 | Sweden | ⤷ Get Started Free | PRODUCT NAME: VEMURAFENIB OCH FARMACEUTISKT GODTAGBARA SALTER DAERAV; REG. NO/DATE: EU/1/12/751/001 20120217 |
| 1893612 | CA 2012 00028 | Denmark | ⤷ Get Started Free | |
| 1893612 | SPC/GB12/021 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: VEMURAFENIB AND PHARMACEUTICALLY ACCEPTABLE SALTS; REGISTERED: UK EU/1/12/751/001 20120221 |
| 1893612 | C300534 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: VEMURAFENIB ALSMEDE FARMACEUTISCH AANVAARDBARE ZOUTEN DAARVAN; REGISTRATION NO/DATE: EU/1/12/751/001 20120217 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for ZELBORAF (Vemurafenib)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
