VELPHORO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Velphoro, and when can generic versions of Velphoro launch?
Velphoro is a drug marketed by Vifor Fresenius and is included in one NDA. There are eleven patents protecting this drug.
This drug has one hundred and nineteen patent family members in thirty-five countries.
The generic ingredient in VELPHORO is ferric oxyhydroxide. There are twenty drug master file entries for this compound. Nine suppliers are listed for this compound. Additional details are available on the ferric oxyhydroxide profile page.
DrugPatentWatch® Generic Entry Outlook for Velphoro
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be July 23, 2030. This may change due to patent challenges or generic licensing.
There have been six patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for VELPHORO
| International Patents: | 119 |
| US Patents: | 11 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 4 |
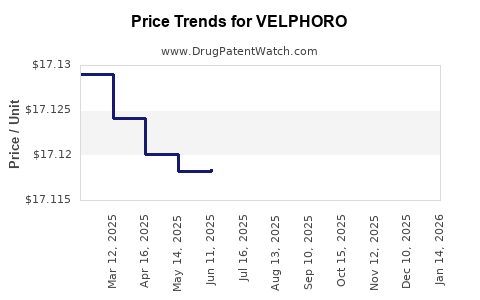
| Drug Prices: | Drug price information for VELPHORO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for VELPHORO |
| What excipients (inactive ingredients) are in VELPHORO? | VELPHORO excipients list |
| DailyMed Link: | VELPHORO at DailyMed |
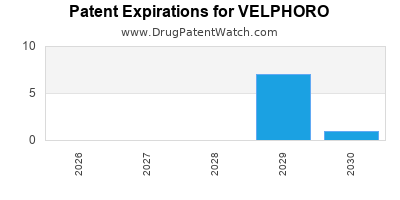
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for VELPHORO
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for VELPHORO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Colorado, Denver | Phase 4 |
| Prim. Priv. Doz. Dr. Daniel Cejka | Phase 2 |
| Vifor Fresenius Medical Care Renal Pharma | Phase 2 |
Pharmacology for VELPHORO
| Drug Class | Phosphate Binder |
| Mechanism of Action | Phosphate Chelating Activity |
US Patents and Regulatory Information for VELPHORO
VELPHORO is protected by eleven US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of VELPHORO is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting VELPHORO
Pharmaceutical composition, comprising phosphate binder particles
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition, comprising phosphate binder particles
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition, comprising phosphate binder particles
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vifor Fresenius | VELPHORO | ferric oxyhydroxide | TABLET, CHEWABLE;ORAL | 205109-001 | Nov 27, 2013 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Vifor Fresenius | VELPHORO | ferric oxyhydroxide | TABLET, CHEWABLE;ORAL | 205109-001 | Nov 27, 2013 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Vifor Fresenius | VELPHORO | ferric oxyhydroxide | TABLET, CHEWABLE;ORAL | 205109-001 | Nov 27, 2013 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Vifor Fresenius | VELPHORO | ferric oxyhydroxide | TABLET, CHEWABLE;ORAL | 205109-001 | Nov 27, 2013 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for VELPHORO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Vifor Fresenius | VELPHORO | ferric oxyhydroxide | TABLET, CHEWABLE;ORAL | 205109-001 | Nov 27, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for VELPHORO
When does loss-of-exclusivity occur for VELPHORO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 9312
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 08322963
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0820308
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 00444
Estimated Expiration: ⤷ Sign Up
China
Patent: 1861146
Estimated Expiration: ⤷ Sign Up
Patent: 4688702
Estimated Expiration: ⤷ Sign Up
Patent: 6619710
Estimated Expiration: ⤷ Sign Up
Patent: 1789820
Estimated Expiration: ⤷ Sign Up
Patent: 2022796
Estimated Expiration: ⤷ Sign Up
Patent: 2022821
Estimated Expiration: ⤷ Sign Up
Patent: 2022822
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 80398
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0170034
Estimated Expiration: ⤷ Sign Up
Patent: 0181837
Estimated Expiration: ⤷ Sign Up
Patent: 0220318
Estimated Expiration: ⤷ Sign Up
Patent: 0230588
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 18496
Estimated Expiration: ⤷ Sign Up
Patent: 21230
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 22285
Estimated Expiration: ⤷ Sign Up
Patent: 43992
Estimated Expiration: ⤷ Sign Up
Patent: 92069
Estimated Expiration: ⤷ Sign Up
Patent: 95699
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 22285
Estimated Expiration: ⤷ Sign Up
Patent: 43992
Estimated Expiration: ⤷ Sign Up
Patent: 92069
Estimated Expiration: ⤷ Sign Up
Patent: 95698
Estimated Expiration: ⤷ Sign Up
Patent: 95699
Estimated Expiration: ⤷ Sign Up
Patent: 95700
Estimated Expiration: ⤷ Sign Up
Finland
Patent: 95699
Estimated Expiration: ⤷ Sign Up
Guatemala
Patent: 1000144
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 07298
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 31293
Estimated Expiration: ⤷ Sign Up
Patent: 41429
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 5726
Estimated Expiration: ⤷ Sign Up
Patent: 7741
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 66164
Estimated Expiration: ⤷ Sign Up
Patent: 61601
Estimated Expiration: ⤷ Sign Up
Patent: 94260
Estimated Expiration: ⤷ Sign Up
Patent: 38734
Estimated Expiration: ⤷ Sign Up
Patent: 64959
Estimated Expiration: ⤷ Sign Up
Patent: 16055
Estimated Expiration: ⤷ Sign Up
Patent: 11503148
Estimated Expiration: ⤷ Sign Up
Patent: 14062120
Estimated Expiration: ⤷ Sign Up
Patent: 15166359
Estimated Expiration: ⤷ Sign Up
Patent: 17114873
Estimated Expiration: ⤷ Sign Up
Patent: 19089805
Estimated Expiration: ⤷ Sign Up
Patent: 21001192
Estimated Expiration: ⤷ Sign Up
Patent: 23011873
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 22285
Estimated Expiration: ⤷ Sign Up
Patent: 43992
Estimated Expiration: ⤷ Sign Up
Patent: 92069
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 6383
Estimated Expiration: ⤷ Sign Up
Patent: 3729
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 10005346
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 898
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 5435
Estimated Expiration: ⤷ Sign Up
Philippines
Patent: 015500727
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 22285
Estimated Expiration: ⤷ Sign Up
Patent: 43992
Estimated Expiration: ⤷ Sign Up
Patent: 92069
Estimated Expiration: ⤷ Sign Up
Patent: 95699
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 22285
Estimated Expiration: ⤷ Sign Up
Patent: 43992
Estimated Expiration: ⤷ Sign Up
Patent: 92069
Estimated Expiration: ⤷ Sign Up
Patent: 95699
Estimated Expiration: ⤷ Sign Up
Patent: 95700
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 93831
Estimated Expiration: ⤷ Sign Up
Patent: 48760
Estimated Expiration: ⤷ Sign Up
Patent: 10124424
Estimated Expiration: ⤷ Sign Up
Patent: 13128356
Estimated Expiration: ⤷ Sign Up
Saudi Arabia
Patent: 2340044
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 8789
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 22285
Estimated Expiration: ⤷ Sign Up
Patent: 43992
Estimated Expiration: ⤷ Sign Up
Patent: 92069
Estimated Expiration: ⤷ Sign Up
Patent: 95699
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1004256
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1438071
Estimated Expiration: ⤷ Sign Up
Patent: 1590115
Estimated Expiration: ⤷ Sign Up
Patent: 100126266
Estimated Expiration: ⤷ Sign Up
Patent: 130030306
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 12331
Estimated Expiration: ⤷ Sign Up
Patent: 03158
Estimated Expiration: ⤷ Sign Up
Patent: 08719
Estimated Expiration: ⤷ Sign Up
Patent: 50337
Estimated Expiration: ⤷ Sign Up
Patent: 51457
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 68167
Estimated Expiration: ⤷ Sign Up
Patent: 92159
Estimated Expiration: ⤷ Sign Up
Patent: 0938210
Estimated Expiration: ⤷ Sign Up
Patent: 1509423
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 10000152
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering VELPHORO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Denmark | 0868125 | ⤷ Sign Up | |
| Spain | 2171758 | ⤷ Sign Up | |
| Portugal | 3073997 | ⤷ Sign Up | |
| Australia | 2008322963 | Pharmaceutical compositions | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VELPHORO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0868125 | 15C0018 | France | ⤷ Sign Up | PRODUCT NAME: MELANGE D'OXYHYDROXYDE DE FER (III) POLYNUCLEAIRE,DE SACCHAROSE ET D'AMIDONS.; REGISTRATION NO/DATE: EU/1/14/943/001 20140826 |
| 0868125 | SPC/GB14/087 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: MIXTURE OF POLYNUCLEAR IRON(III)OXYHYDROXIDE, SUCROSE AND STARCHES; REGISTERED: UK EU/1/14/943/001 20140828; UK EU/1/14/943/002 20140828; UK EU/1/14/943/003 20140828; UK EU/1/14/943/004 20140828 |
| 0868125 | C300710 | Netherlands | ⤷ Sign Up | PRODUCT NAME: MENGSEL VAN POLYNUCLEAIRE IJZER (III)-OXYHYDROXIDE, SUCROSE EN ZETMEEL; REGISTRATION NO/DATE: EU/1/14/943 20140826 |
| 0868125 | 425 | Finland | ⤷ Sign Up | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


