Last updated: July 27, 2025
Introduction
Valproic acid, a well-established anticonvulsant and mood stabilizer, continues to play a pivotal role in the management of neurological and psychiatric conditions. First approved in the 1960s, it remains integral to epilepsy treatment protocols, bipolar disorder management, and off-label uses such as migraine prophylaxis. As the pharmaceutical industry evolves, understanding the market dynamics and financial prospects of valproic acid offers strategic insights for stakeholders ranging from manufacturers to healthcare providers.
Market Overview
The global valproic acid market has historically been characterized by steady growth, driven by its established efficacy, broad therapeutic applications, and expanding patient populations. The market is estimated to be valued at approximately USD 1.4 billion in 2022, with projections indicating a compound annual growth rate (CAGR) of around 4-5% over the next five years.[1]
The core drivers include rising prevalence of epilepsy and bipolar disorder, increased awareness of mental health, and expanding off-label use cases. However, growth is tempered by challenges such as safety concerns, regulatory hurdles, and the emergence of newer therapeutic alternatives.
Key Market Drivers
- Prevalence of Epilepsy and Bipolar Disorder
Epilepsy affects roughly 50 million people worldwide, according to the World Health Organization (WHO), making it one of the most common neurological disorders.[2] Valproic acid remains a frontline therapy, especially for generalized seizures. Similarly, bipolar disorder affects over 60 million individuals globally, with valproic acid considered an effective mood stabilizer.[3]
- Expanding Off-Label Uses
Clinicians increasingly prescribe valproic acid off-label for migraine prophylaxis, neuropathic pain, and other psychiatric conditions. The versatility of the drug sustains its market demand despite emerging alternatives.
- Growing & Aging Population
An aging global population results in higher incidence rates of neurological conditions responsive to valproic acid. The demand for long-term management in elderly patients sustains the drug’s sales.
- Advancements in Formulation and Delivery
Development of extended-release formulations and combination therapies enhances patient adherence and safety, stabilizing its market position.
Market Challenges and Restraints
- Safety and Regulatory Concerns
The thalidomide-like teratogenic risks associated with valproic acid remain paramount. Numerous countries have imposed restrictions on its use during pregnancy, leading to decreased prescribing in women of childbearing age.[4] The FDA issued a Risk Evaluation and Mitigation Strategy (REMS) for valproate, limiting its use in women who could become pregnant unless specific conditions are met.
- Side Effect Profile
Potential adverse effects, including hepatotoxicity, weight gain, and cognitive impairment, deter some prescribers and patients, especially when alternative drugs with better safety profiles are available.
- Emergence of Newer Therapeutics
Novel antiepileptic drugs such as levetiracetam, lacosamide, and brivaracetam offer comparable efficacy with improved safety, impacting valproic acid's market share.
- Patent and Manufacturing Challenges
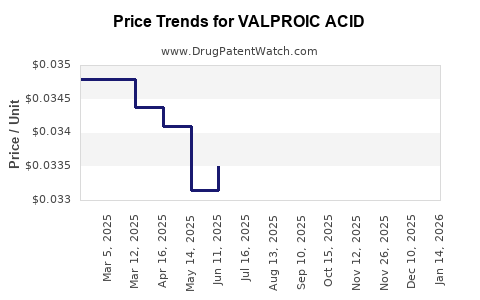
Although valproic acid is off-patent, concerns over manufacturing quality control and generic pricing dynamics affect profitability.
Financial Trajectory and Industry Outlook
Despite the challenges, the financial outlook for valproic acid remains robust due to its entrenched clinical role and the large patient base. The growth is sustained largely through generic markets, with branded versions losing dominance over time. Key prescription markets, such as North America and Europe, show stabilized demand, but emerging markets, including Asia-Pacific and Latin America, demonstrate considerable growth potential owing to increasing healthcare infrastructure.
Manufacturers are also exploring niche applications and combination therapies to diversify revenue streams. For instance, developing controlled-release formulations and adjunct use in specific psychiatric indications could reignite growth trajectories.
Regulatory Landscape and Impact
Regulatory authorities enhance safety labeling and restrict certain populations—particularly women of childbearing age—to balance benefits with risks. Such measures may dampen growth prospects temporarily but are essential for maintaining safety standards. Pharmaceutical companies are investing in educational campaigns and risk management programs to navigate these regulatory confines effectively.
Competitive Environment
The market is predominantly served by generic manufacturers, which exert downward pressure on prices and profit margins. The limited pipeline of novel formulations indicates that future innovation may focus more on drug delivery mechanisms and combination therapies rather than new chemical entities. Strategic alliances and acquisitions aim to expand market share and product portfolio diversification.
Emerging Opportunities
- Personalized Medicine Approaches
Pharmacogenetics research aims to optimize valproic acid dosing and minimize adverse effects, potentially enhancing its safety profile and expanding patient eligibility.
- Regional Market Expansion
Developing healthcare infrastructure and increasing epilepsy awareness in emerging economies create opportunities for market penetration.
- Formulation Innovations
Developing formulations with improved safety and tolerability can address some of the drug’s limitations, thereby supporting sustained sales.
Key Market Players
Major pharmaceutical firms engaged in valproic acid production include Teva Pharmaceuticals, Mylan (now part of Viatris), and Hikma Pharmaceuticals. Their strategic focus largely revolves around manufacturing efficiencies, distribution networks, and ensuring regulatory compliance.
Conclusion
Valproic acid's market dynamics are defined by its long-standing efficacy, widespread application, and a complex safety profile influencing regulatory and prescribing behaviors. While challenges persist—primarily safety concerns and competition from newer therapies—the entrenched clinical utility ensures steady demand, especially in emerging markets. Financial prospects hinge on strategic adaptations, such as formulation improvements, regional expansion, and pharmacogenetics integration.
Key Takeaways
-
Market Stability Amid Challenges: The global valproic acid market remains stable due to its foundational role in epilepsy and bipolar disorder treatment, despite safety issues and competitive pressure.
-
Regulatory Impact: Safety regulations, particularly relating to pregnancy risks, restrict prescribing patterns but also encourage innovation in formulations and risk management strategies.
-
Emerging Markets as Growth Frontiers: Asia-Pacific and Latin America represent growth opportunities driven by increasing neurological disorder prevalence and healthcare development.
-
Innovation Focus: Future growth depends on formulation advances, personalized medicine, and strategic patent management, especially in regions with high generic penetration.
-
Competitive Landscape: Dominated by generic manufacturers, the market emphasizes cost-efficiency, regulatory compliance, and expanding indications.
FAQs
-
What are the main therapeutic applications of valproic acid?
It is primarily used for epilepsy, bipolar disorder, and migraine prophylaxis, with off-label uses including neuropathic pain and psychiatric conditions.
-
How do safety concerns influence valproic acid's market trends?
Safety risks, especially teratogenicity, have led to regulatory restrictions and lower prescribing rates among women of childbearing age, impacting sales and encouraging safer alternatives.
-
Are there new formulations of valproic acid in development?
Yes, extended-release formulations and combination therapies are under development to improve safety profiles and patient adherence.
-
What is the outlook for valproic acid in emerging markets?
Significant growth potential exists as healthcare infrastructure improves, and awareness of neurological disorders increases, coupled with the availability of cost-effective generic options.
-
Can personalized medicine enhance the use of valproic acid?
Pharmacogenetics and tailored dosing can optimize efficacy while reducing adverse effects, potentially broadening its safe use and market longevity.
References
[1] Grand View Research. Valproic Acid Market Size, Share & Trends Analysis Report. 2022.
[2] World Health Organization. Epilepsy Fact Sheet. 2021.
[3] American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 2013.
[4] U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies (REMS) for Valproate. 2018.