TRIUMEQ Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Triumeq, and when can generic versions of Triumeq launch?
Triumeq is a drug marketed by Viiv Hlthcare and is included in two NDAs. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and fifty-seven patent family members in thirty-five countries.
The generic ingredient in TRIUMEQ is abacavir sulfate; dolutegravir sodium; lamivudine. There are twelve drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the abacavir sulfate; dolutegravir sodium; lamivudine profile page.
DrugPatentWatch® Generic Entry Outlook for Triumeq
Triumeq was eligible for patent challenges on August 12, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 8, 2030. This may change due to patent challenges or generic licensing.
There have been twenty-five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TRIUMEQ?
- What are the global sales for TRIUMEQ?
- What is Average Wholesale Price for TRIUMEQ?
Summary for TRIUMEQ
| International Patents: | 157 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 2 |
| Clinical Trials: | 20 |
| Drug Prices: | Drug price information for TRIUMEQ |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TRIUMEQ |
| What excipients (inactive ingredients) are in TRIUMEQ? | TRIUMEQ excipients list |
| DailyMed Link: | TRIUMEQ at DailyMed |
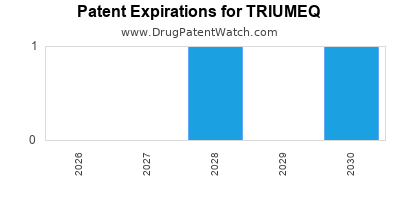
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TRIUMEQ
Generic Entry Date for TRIUMEQ*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for TRIUMEQ
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Macquarie University, Australia | PHASE3 |
| Stichting TRICALS Foundation | Phase 3 |
| King's College London | Phase 3 |
Pharmacology for TRIUMEQ
Paragraph IV (Patent) Challenges for TRIUMEQ
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TRIUMEQ | Tablets | abacavir sulfate; dolutegravir sodium; lamivudine | 600 mg/50 mg/ 300 mg | 205551 | 5 | 2017-08-14 |
US Patents and Regulatory Information for TRIUMEQ
TRIUMEQ is protected by two US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TRIUMEQ is ⤷ Get Started Free.
This potential generic entry date is based on patent 9,242,986.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viiv Hlthcare | TRIUMEQ | abacavir sulfate; dolutegravir sodium; lamivudine | TABLET;ORAL | 205551-001 | Aug 22, 2014 | RX | Yes | Yes | 8,129,385*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Viiv Hlthcare | TRIUMEQ PD | abacavir sulfate; dolutegravir sodium; lamivudine | TABLET, FOR SUSPENSION;ORAL | 215413-001 | Mar 30, 2022 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Viiv Hlthcare | TRIUMEQ | abacavir sulfate; dolutegravir sodium; lamivudine | TABLET;ORAL | 205551-001 | Aug 22, 2014 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TRIUMEQ
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Viiv Hlthcare | TRIUMEQ | abacavir sulfate; dolutegravir sodium; lamivudine | TABLET;ORAL | 205551-001 | Aug 22, 2014 | 5,905,082*PED | ⤷ Get Started Free |
| Viiv Hlthcare | TRIUMEQ | abacavir sulfate; dolutegravir sodium; lamivudine | TABLET;ORAL | 205551-001 | Aug 22, 2014 | 6,294,540*PED | ⤷ Get Started Free |
| Viiv Hlthcare | TRIUMEQ | abacavir sulfate; dolutegravir sodium; lamivudine | TABLET;ORAL | 205551-001 | Aug 22, 2014 | 6,417,191*PED | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TRIUMEQ
When does loss-of-exclusivity occur for TRIUMEQ?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09325128
Estimated Expiration: ⤷ Get Started Free
Patent: 14277831
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0923217
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 44019
Estimated Expiration: ⤷ Get Started Free
Patent: 55957
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2245182
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 76080
Estimated Expiration: ⤷ Get Started Free
Patent: 10603
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 43626
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 86478
Estimated Expiration: ⤷ Get Started Free
Patent: 48595
Estimated Expiration: ⤷ Get Started Free
Patent: 30891
Estimated Expiration: ⤷ Get Started Free
Patent: 12131791
Patent: SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND THEIR INTERMEDIATES
Estimated Expiration: ⤷ Get Started Free
Patent: 12511573
Estimated Expiration: ⤷ Get Started Free
Patent: 16041727
Patent: カルバモイルピリドンHIVインテグラーゼ阻害剤及びそれらの中間体の合成 (SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND THEIR INTERMEDIATES)
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 1942
Patent: SINTESIS DE INHIBIDORES DE INTEGRASA DE VIH DE CARBAMOIL-PIRIDONA E INTERMEDIARIOS. (SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES.)
Estimated Expiration: ⤷ Get Started Free
Patent: 3683
Patent: SINTESIS DE INHIBIDORES DE INTEGRASA DE VIH DE CARBAMOIL-PIRIDONA E INTERMEDIARIOS. (SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES)
Estimated Expiration: ⤷ Get Started Free
Patent: 11006241
Patent: SINTESIS DE INHIBIDORES DE INTEGRASA DE VIH DE CARBAMOIL-PIRIDONA E INTERMEDIARIOS. (SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES.)
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 27451
Patent: СИНТЕЗ КАРБАМОИЛПИРИДОНОВЫХ ИНГИБИТОРОВ ИНТЕГРАЗЫ ВИЧ И ПРОМЕЖУТОЧНЫХ СОЕДИНЕНИЙ (SYNTHESIS OF CARBAMOYL PYRIDONE INHIBITORS OF HIV INTEGRASE AND INTERMEDIATE COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 38923
Patent: Синтез карбамоилпиридоновых ингибиторов интегразы ВИЧ и промежуточных соединений (SYNTHESIS OF CARBAMOIL-PYRIDONE INHIBITORS OF HIV INTEGRASE AND INTERMEDIATE COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 11121785
Patent: СИНТЕЗ КАРБАМОИЛПИРИДОНОВЫХ ИНГИБИТОРОВ ИНТЕГРАЗЫ ВИЧ И ПРОМЕЖУТОЧНЫХ СОЕДИНЕНИЙ
Estimated Expiration: ⤷ Get Started Free
Patent: 13153004
Patent: СИНТЕЗ КАРБАМОИЛПИРИДОНОВЫХ ИНГИБИТОРОВ ИНТЕГРАЗЫ ВИЧ И ПРОМЕЖУТОЧНЫХ СОЕДИНЕНИЙ
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 1308
Patent: SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1733625
Estimated Expiration: ⤷ Get Started Free
Patent: 1847887
Estimated Expiration: ⤷ Get Started Free
Patent: 110094336
Patent: SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES
Estimated Expiration: ⤷ Get Started Free
Patent: 170038116
Patent: 카르바모일피리돈 HIV 인테그라제 억제제 및 중간체의 합성 (SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 41765
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 83947
Estimated Expiration: ⤷ Get Started Free
Patent: 1030010
Patent: Synthesis of carbamoylpyridone HIV integrase inhibitors and intermediates
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TRIUMEQ around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Germany | 122005000029 | ⤷ Get Started Free | |
| World Intellectual Property Organization (WIPO) | 2007049675 | ⤷ Get Started Free | |
| Australia | 2014277831 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TRIUMEQ
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0817637 | 05C0022 | France | ⤷ Get Started Free | PRODUCT NAME: ABACAVIR SULFATE; LAMIVUDINE; REGISTRATION NO/DATE: EU/1/04/298/001 20041217 |
| 1874117 | 14C0041 | France | ⤷ Get Started Free | PRODUCT NAME: DOLUTEGRAVIR ET SES SELS OU SOLVATES PHARMACEUTIQUEMENT ACCEPTABLES,NOTAMMENT LE DOLUTEGRAVIR SODIQUE.; REGISTRATION NO/DATE: EU/1/13/892/001-002 20140121 |
| 2465580 | 21C1023 | France | ⤷ Get Started Free | PRODUCT NAME: CABOTEGRAVIR OU L'UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; NAT. REGISTRATION NO/DATE: EU/1/20/1481 20201221; FIRST REGISTRATION: - EU/1/20/1481 20201221 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for TRIUMEQ
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
