Last updated: July 28, 2025
Introduction
Tenofovir disoproxil fumarate (TDF) is a cornerstone in the treatment of HIV/AIDS and hepatitis B virus (HBV) infections. Launched in the early 2000s, it revolutionized antiviral therapy with its potent efficacy, favorable safety profile, and once-daily dosing. Over the years, TDF's market dynamics have experienced significant shifts driven by patent expirations, generics entry, evolving treatment guidelines, and the advent of newer agents. This analysis delineates the current landscape, key market forces, and future financial trajectories shaping TDF's role in global healthcare.
Pharmacological Profile and Clinical Application
TDF is a prodrug of tenofovir, a nucleotide reverse transcriptase inhibitor (NRTI). It exerts antiviral activity by inhibiting HIV reverse transcriptase and HBV DNA polymerase. Its once-daily oral administration enhances adherence, and its relatively benign safety profile—pending monitoring for renal and bone health—has established it as a first-line agent in antiretroviral regimens [1].
Market Landscape: Key Drivers
-
Patent Expiry and Generic Penetration:
Gilead Sciences' original TDF formulation, Viread, gained FDA approval in 2001. Its patent expiration in many jurisdictions—primarily around 2018-2020—has precipitated a surge in generic versions, sharply reducing pricing. Generic manufacturers have expanded access, especially in low- and middle-income countries, aiding global HIV management efforts.
-
Treatment Guidelines and Clinical Preferences:
Leading authorities, such as the WHO and Department of Health and Human Services (DHHS), have initially included TDF as a cornerstone, but recent guidelines are shifting. The adoption of tenofovir alafenamide (TAF)—a prodrug with improved safety—has prompted clinicians to favor TAF over TDF in some cases, influencing market share and revenue streams.
-
Regulatory Approvals and Patent Challenges:
The entry of biosimilars and generics has created price competition. Ongoing patent litigation and patent extensions, like data exclusivities, complicate timing but generally allow generics to dominate markets within a decade of initial approval.
-
Disease Epidemiology and Access:
The global HIV epidemic persists, with approximately 38 million people living with HIV/AIDS [2]. TDF remains vital, especially in resource-limited settings, driven by its affordability and efficacy. Concurrently, HBV treatment uses TDF extensively, contributing additional revenue streams.
Financial Trajectory: Historical and Projected
-
Pre-Patent Expiry (2001–2018):
During this period, Gilead's Viread generated peak revenues, with estimated sales reaching over $3 billion annually. Margins were high due to patent protection and limited competition.
-
Post-Patent Era (2018–2023):
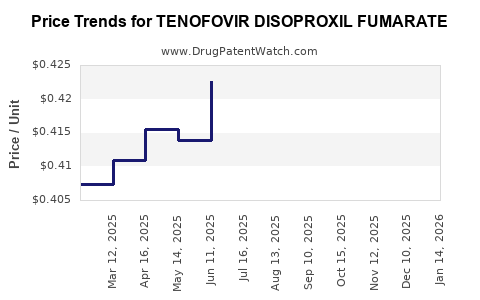
The patent cliff catalyzed a rapid decline in branded sales, with generics capturing significant market share worldwide. Prices dropped by over 90% in some regions, with global sales declining accordingly. However, volume increases in emerging markets partially offset price reductions, sustaining overall revenues at a lower but stable level.
-
Current Market Size and Forecast (2024–2030):
The global TDF market is projected to stabilize at approximately $800 million to $1 billion annually, predominantly driven by generic sales, with substantial commercial presence in Africa, Southeast Asia, and Latin America.
The continued adoption of fixed-dose combination (FDC) products incorporating TDF, such as Triumeq and others, sustains demand. Nonetheless, the rapid ascendancy of TAF-based regimens portends a gradual decline in TDF’s dominance over the next decade, especially in high-income regions.
-
Emerging Trends Impacting Financials:
- Shift to TAF: With its improved bone and renal safety, TAF's market penetration is expected to displace TDF in many developed settings, reducing TDF's revenue share.
- Development of Novel Agents: Future antiviral innovations targeting HIV and HBV could further erode TDF's market share.
- Global Health Initiatives: Pooled procurement and generic manufacturing in low-income countries are instrumental in maintaining access but suppress pricing and margins.
Market Challenges and Opportunities
-
Challenges:
- The decline of patent protections expediting generics proliferation.
- Competition from newer agents offering improved safety profiles.
- Regulatory delays or barriers in emerging markets.
- Patent disputes over formulations and delivery mechanisms.
-
Opportunities:
- Development of fixed-dose combinations leveraging TDF, TAF, and other agents to improve treatment adherence.
- Expansion into pediatric, co-morbid, or multi-morbidity treatment paradigms.
- Strategic licensing and partnerships in developing countries.
- Capitalizing on global health funding and initiatives prioritizing HIV/HBV treatment.
Strategic Outlook
Pharmaceutical companies with rights to TDF assets should anticipate continued revenue from legacy markets but prepare for a proactive transition toward TAF-based therapies and innovative compounds. Investment in biosimilar manufacturing and regional manufacturing capabilities can enhance market penetration, especially as prices slide due to generic competition.
In parallel, patent litigation and regulatory pathways will significantly influence the timing and scope of future revenue streams. Approaches involving rights extension through orphan drug designations or formulation innovations could create alternative market opportunities. Overall, the TDF market exemplifies the lifecycle progression of a first-generation antiviral, with future income prospects increasingly tied to generic manufacturing, geographic expansion, and product line diversification.
Regulatory and Patent Landscape
Major patent protections for Viread have expired in key markets, including the U.S. and EU. Gilead's patent estate faced challenges over formulation patents, with courts granting or invalidating key patents in different jurisdictions [3]. Regulatory agencies have approved multiple generic versions, further accelerating market commoditization.
Key COVID-19 Impact
While not directly affecting TDF, the COVID-19 pandemic impacted supply chains, delayed regulatory reviews, and shifted healthcare priorities worldwide. Increased focus on antiviral research, with some interest in repurposing TDF against emerging viruses, remains a tentative area of investigation but currently has minimal impact on standard market trajectories.
Conclusion
The market for tenofovir disoproxil fumarate is transitioning from patent-protected blockbuster status to an era defined by generic competition and evolving treatment preferences. While its revenue contribution diminishes in high-income markets favoring TAF, TDF remains indispensable in resource-limited settings. Strategic positioning, innovations, and market expansion in underserved regions are pivotal for stakeholders aiming to optimize future financial outcomes.
Key Takeaways
- Patent expiration precipitated a significant decline in TDF revenues, with generics dominating global markets.
- Transition to tenofovir alafenamide (TAF) is shifting clinician and patient preferences, especially in developed countries.
- TDF's future revenue is primarily derived from low-cost generics in emerging markets and fixed-dose combinations.
- Patent challenges and regulatory approvals influence the timeline and extent of generic market penetration.
- Companies should focus on geographic expansion, formulation innovations, and diversification to sustain profitability in the evolving TDF landscape.
FAQs
1. How has patent expiry affected TDF's market share?
Patent expiry has led to the aggressive entry of generic manufacturers, drastically reducing TDF's prices and revenues in many regions while expanding access in developing countries. The original branded versions saw a sharp decline in market share post-patent expiration.
2. What role does TAF play in the current TDF market?
TAF offers comparable efficacy with improved safety profiles. It is gradually replacing TDF in many developed markets, thereby reducing TDF’s market share in these areas. TAF-based regimens are increasingly preferred in high-income settings.
3. Is TDF still relevant in HIV treatment globally?
Yes, particularly in low- and middle-income countries, where cost-effective generic TDF remains a critical component of HIV treatment programs. Its affordability and efficacy sustain its relevance despite competition from newer agents.
4. What are the key factors influencing TDF's future revenues?
Patent status, emerging treatment guidelines favoring TAF, market access in developing regions, regulatory approvals of generics, and ongoing development of combination therapies are primary factors.
5. How might future innovations impact TDF's financial prospects?
Innovations such as novel formulations, combination products, or new antiviral agents could diminish TDF’s market share further, emphasizing the importance of strategic diversification for stakeholders.
References
[1] Gilead Sciences. Viread (tenofovir disoproxil fumarate) Prescribing Information. 2022.
[2] UNAIDS. Global HIV & AIDS statistics — 2022 fact sheet.
[3] U.S. Patent and Trademark Office. Patent disputes and legal challenges related to tenofovir formulations.