SOVALDI Drug Patent Profile
✉ Email this page to a colleague
When do Sovaldi patents expire, and what generic alternatives are available?
Sovaldi is a drug marketed by Gilead Sciences Inc and is included in two NDAs. There are nine patents protecting this drug and one Paragraph IV challenge.
This drug has three hundred and sixty-eight patent family members in forty-nine countries.
The generic ingredient in SOVALDI is sofosbuvir. There are nine drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the sofosbuvir profile page.
DrugPatentWatch® Generic Entry Outlook for Sovaldi
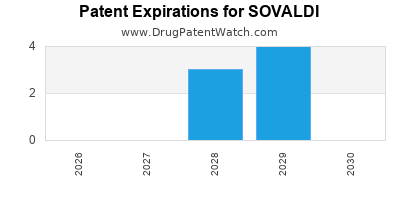
Sovaldi was eligible for patent challenges on December 6, 2017.
There have been four patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for SOVALDI?
- What are the global sales for SOVALDI?
- What is Average Wholesale Price for SOVALDI?
Summary for SOVALDI
| International Patents: | 368 |
| US Patents: | 9 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 49 |
| Clinical Trials: | 34 |
| Drug Prices: | Drug price information for SOVALDI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for SOVALDI |
| What excipients (inactive ingredients) are in SOVALDI? | SOVALDI excipients list |
| DailyMed Link: | SOVALDI at DailyMed |

Recent Clinical Trials for SOVALDI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Cairo University | Phase 3 |
| Ain Shams University | Phase 3 |
| Assiut University | Early Phase 1 |
Pharmacology for SOVALDI
| Drug Class | Hepatitis C Virus Nucleotide Analog NS5B Polymerase Inhibitor |
| Mechanism of Action | RNA Replicase Inhibitors |
Paragraph IV (Patent) Challenges for SOVALDI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SOVALDI | Tablets | sofosbuvir | 400 mg | 204671 | 2 | 2017-12-06 |
US Patents and Regulatory Information for SOVALDI
SOVALDI is protected by nine US patents and two FDA Regulatory Exclusivities.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gilead Sciences Inc | SOVALDI | sofosbuvir | TABLET;ORAL | 204671-001 | Dec 6, 2013 | RX | Yes | Yes | 8,618,076*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Gilead Sciences Inc | SOVALDI | sofosbuvir | TABLET;ORAL | 204671-001 | Dec 6, 2013 | RX | Yes | Yes | 8,580,765*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Gilead Sciences Inc | SOVALDI | sofosbuvir | TABLET;ORAL | 204671-001 | Dec 6, 2013 | RX | Yes | Yes | 8,889,159*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Gilead Sciences Inc | SOVALDI | sofosbuvir | TABLET;ORAL | 204671-001 | Dec 6, 2013 | RX | Yes | Yes | 7,964,580*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Gilead Sciences Inc | SOVALDI | sofosbuvir | PELLETS;ORAL | 212480-001 | Aug 28, 2019 | RX | Yes | No | 8,334,270*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for SOVALDI
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Gilead Sciences Ireland UC | Sovaldi | sofosbuvir | EMEA/H/C/002798Sovaldi is indicated in combination with other medicinal products for the treatment of chronic hepatitis C (CHC) in adult and paediatric patients aged 3 years and above (see sections 4.2, 4.4 and 5.1).For hepatitis C virus (HCV) genotype specific activity, see sections 4.4 and 5.1.Sovaldi is indicated in combination with other medicinal products for the treatment of chronic hepatitis C (CHC) in adults and paediatric patients aged 3 years and above (see sections 4.2, 4.4 and 5.1).For hepatitis C virus (HCV) genotype specific activity, see sections 4.4 and 5.1. | Authorised | no | no | no | 2014-01-16 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for SOVALDI
When does loss-of-exclusivity occur for SOVALDI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 15
Estimated Expiration: ⤷ Get Started Free
Argentina
Patent: 0819
Estimated Expiration: ⤷ Get Started Free
Patent: 0870
Estimated Expiration: ⤷ Get Started Free
Patent: 1813
Estimated Expiration: ⤷ Get Started Free
Patent: 2937
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 10249481
Estimated Expiration: ⤷ Get Started Free
Patent: 11235044
Estimated Expiration: ⤷ Get Started Free
Patent: 11235112
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 1012781
Estimated Expiration: ⤷ Get Started Free
Patent: 2012024884
Estimated Expiration: ⤷ Get Started Free
Patent: 2012024923
Estimated Expiration: ⤷ Get Started Free
Patent: 2013004621
Estimated Expiration: ⤷ Get Started Free
Patent: 2013007556
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 63151
Estimated Expiration: ⤷ Get Started Free
Patent: 94669
Estimated Expiration: ⤷ Get Started Free
Patent: 94671
Estimated Expiration: ⤷ Get Started Free
Patent: 19700
Estimated Expiration: ⤷ Get Started Free
Patent: 49694
Estimated Expiration: ⤷ Get Started Free
Patent: 15187
Estimated Expiration: ⤷ Get Started Free
Patent: 88217
Estimated Expiration: ⤷ Get Started Free
Patent: 77960
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 10000520
Estimated Expiration: ⤷ Get Started Free
Patent: 11000716
Estimated Expiration: ⤷ Get Started Free
Patent: 11000717
Estimated Expiration: ⤷ Get Started Free
Patent: 11000718
Estimated Expiration: ⤷ Get Started Free
Patent: 13000903
Estimated Expiration: ⤷ Get Started Free
Patent: 13000904
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2459299
Estimated Expiration: ⤷ Get Started Free
Patent: 2858790
Estimated Expiration: ⤷ Get Started Free
Patent: 2906102
Estimated Expiration: ⤷ Get Started Free
Patent: 4017020
Estimated Expiration: ⤷ Get Started Free
Patent: 4292256
Estimated Expiration: ⤷ Get Started Free
Patent: 5085592
Estimated Expiration: ⤷ Get Started Free
Patent: 5198949
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 70789
Estimated Expiration: ⤷ Get Started Free
Patent: 30166
Estimated Expiration: ⤷ Get Started Free
Patent: 30167
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 120532
Estimated Expiration: ⤷ Get Started Free
Patent: 120534
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0151075
Estimated Expiration: ⤷ Get Started Free
Patent: 0160958
Estimated Expiration: ⤷ Get Started Free
Patent: 0171267
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 16976
Estimated Expiration: ⤷ Get Started Free
Patent: 18045
Estimated Expiration: ⤷ Get Started Free
Patent: 19273
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 32792
Estimated Expiration: ⤷ Get Started Free
Patent: 52930
Estimated Expiration: ⤷ Get Started Free
Patent: 09923
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 12012282
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 6341
Estimated Expiration: ⤷ Get Started Free
Patent: 6731
Estimated Expiration: ⤷ Get Started Free
Patent: 8709
Estimated Expiration: ⤷ Get Started Free
Patent: 8742
Estimated Expiration: ⤷ Get Started Free
Patent: 1171417
Estimated Expiration: ⤷ Get Started Free
Patent: 1290988
Estimated Expiration: ⤷ Get Started Free
Patent: 1290993
Estimated Expiration: ⤷ Get Started Free
Patent: 1370186
Estimated Expiration: ⤷ Get Started Free
Patent: 1592101
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 32792
Estimated Expiration: ⤷ Get Started Free
Patent: 52930
Estimated Expiration: ⤷ Get Started Free
Patent: 52931
Estimated Expiration: ⤷ Get Started Free
Patent: 52933
Estimated Expiration: ⤷ Get Started Free
Patent: 09923
Estimated Expiration: ⤷ Get Started Free
Patent: 10264
Estimated Expiration: ⤷ Get Started Free
Patent: 52422
Estimated Expiration: ⤷ Get Started Free
Patent: 10562
Estimated Expiration: ⤷ Get Started Free
Patent: 13337
Estimated Expiration: ⤷ Get Started Free
Patent: 90428
Estimated Expiration: ⤷ Get Started Free
Patent: 21275
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 69414
Estimated Expiration: ⤷ Get Started Free
Patent: 78171
Estimated Expiration: ⤷ Get Started Free
Patent: 81775
Estimated Expiration: ⤷ Get Started Free
Patent: 99645
Estimated Expiration: ⤷ Get Started Free
Patent: 13571
Estimated Expiration: ⤷ Get Started Free
Patent: 13572
Estimated Expiration: ⤷ Get Started Free
Patent: 17494
Estimated Expiration: ⤷ Get Started Free
Patent: 19106
Estimated Expiration: ⤷ Get Started Free
Patent: 51578
Estimated Expiration: ⤷ Get Started Free
Patent: 54977
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 26235
Estimated Expiration: ⤷ Get Started Free
Patent: 31637
Estimated Expiration: ⤷ Get Started Free
Patent: 34239
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 6492
Estimated Expiration: ⤷ Get Started Free
Patent: 2099
Estimated Expiration: ⤷ Get Started Free
Patent: 2174
Estimated Expiration: ⤷ Get Started Free
Patent: 7471
Estimated Expiration: ⤷ Get Started Free
Patent: 9115
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 72539
Estimated Expiration: ⤷ Get Started Free
Patent: 85659
Estimated Expiration: ⤷ Get Started Free
Patent: 09535
Estimated Expiration: ⤷ Get Started Free
Patent: 58528
Estimated Expiration: ⤷ Get Started Free
Patent: 06716
Estimated Expiration: ⤷ Get Started Free
Patent: 55605
Estimated Expiration: ⤷ Get Started Free
Patent: 12527477
Estimated Expiration: ⤷ Get Started Free
Patent: 13523767
Estimated Expiration: ⤷ Get Started Free
Patent: 13525277
Estimated Expiration: ⤷ Get Started Free
Patent: 13527145
Estimated Expiration: ⤷ Get Started Free
Patent: 15028060
Estimated Expiration: ⤷ Get Started Free
Patent: 15205903
Estimated Expiration: ⤷ Get Started Free
Patent: 16053045
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 32792
Estimated Expiration: ⤷ Get Started Free
Patent: 09923
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 6918
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 0725
Estimated Expiration: ⤷ Get Started Free
Patent: 11012417
Estimated Expiration: ⤷ Get Started Free
Patent: 12011171
Estimated Expiration: ⤷ Get Started Free
Patent: 12011324
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 294
Estimated Expiration: ⤷ Get Started Free
Patent: 846
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 6635
Estimated Expiration: ⤷ Get Started Free
Patent: 3232
Estimated Expiration: ⤷ Get Started Free
Patent: 3602
Estimated Expiration: ⤷ Get Started Free
Patent: 9926
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 130151
Estimated Expiration: ⤷ Get Started Free
Patent: 130183
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 014502684
Estimated Expiration: ⤷ Get Started Free
Patent: 015502237
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 32792
Estimated Expiration: ⤷ Get Started Free
Patent: 52930
Estimated Expiration: ⤷ Get Started Free
Patent: 09923
Estimated Expiration: ⤷ Get Started Free
Patent: 90428
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 32792
Estimated Expiration: ⤷ Get Started Free
Patent: 52930
Estimated Expiration: ⤷ Get Started Free
Patent: 09923
Estimated Expiration: ⤷ Get Started Free
Patent: 52422
Estimated Expiration: ⤷ Get Started Free
Patent: 13337
Estimated Expiration: ⤷ Get Started Free
Patent: 90428
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01500285
Estimated Expiration: ⤷ Get Started Free
Patent: 01600316
Patent: E PROCEDIMENTO PER LA PREPARAZIONE DELL'ESTERE 1-METILETILICO DI N-[(2'R)- 2'-DEOSSI-2'-FLUORO-2'-METIL-P-FENIL-5'- URIDILlL]-L-ALANINA
Estimated Expiration: ⤷ Get Started Free
Patent: 01700412
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 368
Estimated Expiration: ⤷ Get Started Free
Patent: 229
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 6197
Estimated Expiration: ⤷ Get Started Free
Patent: 4323
Estimated Expiration: ⤷ Get Started Free
Patent: 4324
Estimated Expiration: ⤷ Get Started Free
Patent: 201500835W
Estimated Expiration: ⤷ Get Started Free
Patent: 201702025S
Estimated Expiration: ⤷ Get Started Free
Patent: 201702294Q
Estimated Expiration: ⤷ Get Started Free
Patent: 201708263S
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 32792
Estimated Expiration: ⤷ Get Started Free
Patent: 52930
Estimated Expiration: ⤷ Get Started Free
Patent: 09923
Estimated Expiration: ⤷ Get Started Free
Patent: 90428
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1108749
Patent: N-[(2'R)-2'-DEOXY-2'-FLUORO-2'-METHYL-P-PHENYL-5'-URIDYLYL]-ALANINE 1 -METHYLETHYL ESTER AND PROCESS FOR ITS PRODUCTION
Estimated Expiration: ⤷ Get Started Free
Patent: 1207799
Patent: NUCLEOSIDE PHOSPHORAMIDATES
Estimated Expiration: ⤷ Get Started Free
Patent: 1207800
Patent: STEREOSELECTIVE SYNTHESIS OF PHOSPHORUS CONTAINING ACTIVES
Estimated Expiration: ⤷ Get Started Free
Patent: 1400249
Patent: NUCLEOSIDE PHOSPHORAMIDATES
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1599183
Estimated Expiration: ⤷ Get Started Free
Patent: 1603817
Estimated Expiration: ⤷ Get Started Free
Patent: 1715981
Estimated Expiration: ⤷ Get Started Free
Patent: 1759369
Estimated Expiration: ⤷ Get Started Free
Patent: 120034662
Estimated Expiration: ⤷ Get Started Free
Patent: 120138242
Estimated Expiration: ⤷ Get Started Free
Patent: 130064064
Estimated Expiration: ⤷ Get Started Free
Patent: 140147144
Estimated Expiration: ⤷ Get Started Free
Patent: 150043553
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 16466
Estimated Expiration: ⤷ Get Started Free
Patent: 51944
Estimated Expiration: ⤷ Get Started Free
Patent: 86821
Estimated Expiration: ⤷ Get Started Free
Patent: 38350
Estimated Expiration: ⤷ Get Started Free
Patent: 44990
Estimated Expiration: ⤷ Get Started Free
Patent: 48803
Estimated Expiration: ⤷ Get Started Free
Patent: 00773
Estimated Expiration: ⤷ Get Started Free
Patent: 27501
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 98117
Estimated Expiration: ⤷ Get Started Free
Patent: 76352
Estimated Expiration: ⤷ Get Started Free
Patent: 83692
Estimated Expiration: ⤷ Get Started Free
Patent: 98358
Estimated Expiration: ⤷ Get Started Free
Patent: 1107341
Patent: Nucleoside phosphoramidates
Estimated Expiration: ⤷ Get Started Free
Patent: 1136593
Patent: Nucleoside phosphoramidates
Estimated Expiration: ⤷ Get Started Free
Patent: 1136945
Patent: Purine nucleoside phosphoramidate
Estimated Expiration: ⤷ Get Started Free
Patent: 1139457
Patent: Stereoselective synthesis of phosphorus containing actives
Estimated Expiration: ⤷ Get Started Free
Patent: 1518313
Patent: Nucleoside phosphoramidates
Estimated Expiration: ⤷ Get Started Free
Patent: 1704249
Patent: Nucleoside phosphoramidates
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 2959
Patent: НУКЛЕОЗИДФОСФОРАМІДАТИ
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 310
Patent: SINTESIS ESTEREOSELECTIVA DE ACTIVOS QUE CONTIENEN FOSFORO
Estimated Expiration: ⤷ Get Started Free
Patent: 311
Patent: FOSFORAMIDATOS DE NUCLEOSIDOS
Estimated Expiration: ⤷ Get Started Free
Patent: 312
Patent: FOSFORAMIDATO DE NUCLEOSIDO DE PURINA
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering SOVALDI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Denmark | 2552930 | ⤷ Get Started Free | |
| Philippines | 12015502237 | ⤷ Get Started Free | |
| European Patent Office | 3777867 | COMPOSITION ET PROCÉDÉS POUR LE TRAITEMENT DU VIRUS DE L'HÉPATITE C (COMPOSITION AND METHODS FOR TREATING HEPATITIS C VIRUS) | ⤷ Get Started Free |
| Japan | 6073897 | ⤷ Get Started Free | |
| Chile | 2011000718 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SOVALDI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2203462 | C20140035 | Estonia | ⤷ Get Started Free | PRODUCT NAME: SOFOSBUVIIR;REG NO/DATE: EU/1/13/894 17.01.2014 |
| 2430014 | 2016C/006 | Belgium | ⤷ Get Started Free | PRODUCT NAME: LEDIPASVIR/SOFOSBUVIR; AUTHORISATION NUMBER AND DATE: EU/1/14/958 20141118 |
| 2203462 | C 2014 043 | Romania | ⤷ Get Started Free | PRODUCT NAME: SOFOSBUVIR; NATIONAL AUTHORISATION NUMBER: EU/1/13/894(001-002); DATE OF NATIONAL AUTHORISATION: 20140116; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/894(001-002); DATE OF FIRST AUTHORISATION IN EEA: 20140116 |
| 2203462 | 132014902310732 | Italy | ⤷ Get Started Free | PRODUCT NAME: SOFOSBUVIR(SOVALDI); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/13/894, 20140117 |
| 2203462 | 2014/065 | Ireland | ⤷ Get Started Free | PRODUCT NAME: SOVALDI (SOFOSBUVIR); REGISTRATION NO/DATE: EU/1/13/894/001-EU/1/13/894/002 20140116 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory of Sovaldi (sofosbuvir)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
