SAVAYSA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Savaysa, and when can generic versions of Savaysa launch?
Savaysa is a drug marketed by Daiichi Sankyo Inc and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and one patent family members in thirty-four countries.
The generic ingredient in SAVAYSA is edoxaban tosylate. There are four drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the edoxaban tosylate profile page.
DrugPatentWatch® Generic Entry Outlook for Savaysa
Savaysa was eligible for patent challenges on January 8, 2019.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 28, 2028. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for SAVAYSA?
- What are the global sales for SAVAYSA?
- What is Average Wholesale Price for SAVAYSA?
Summary for SAVAYSA
| International Patents: | 101 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 11 |
| Patent Applications: | 358 |
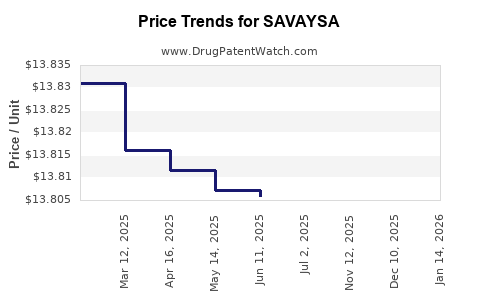
| Drug Prices: | Drug price information for SAVAYSA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for SAVAYSA |
| What excipients (inactive ingredients) are in SAVAYSA? | SAVAYSA excipients list |
| DailyMed Link: | SAVAYSA at DailyMed |
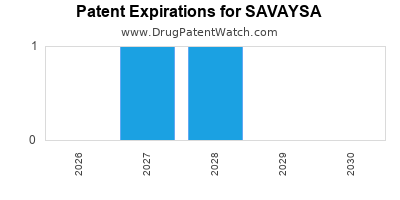
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for SAVAYSA
Generic Entry Date for SAVAYSA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for SAVAYSA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Population Health Research Institute | Phase 3 |
| Johannes Wesling Klinikum Minden | Phase 3 |
| Northumbria Healthcare NHS Foundation Trust | Phase 3 |
Pharmacology for SAVAYSA
| Drug Class | Factor Xa Inhibitor |
| Mechanism of Action | Factor Xa Inhibitors |
Paragraph IV (Patent) Challenges for SAVAYSA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SAVAYSA | Tablets | edoxaban tosylate | 15 mg, 30 mg and 60 mg | 206316 | 1 | 2019-01-28 |
US Patents and Regulatory Information for SAVAYSA
SAVAYSA is protected by two US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of SAVAYSA is ⤷ Get Started Free.
This potential generic entry date is based on patent 9,149,532.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daiichi Sankyo Inc | SAVAYSA | edoxaban tosylate | TABLET;ORAL | 206316-001 | Jan 8, 2015 | RX | Yes | No | 7,365,205 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Daiichi Sankyo Inc | SAVAYSA | edoxaban tosylate | TABLET;ORAL | 206316-003 | Jan 8, 2015 | RX | Yes | Yes | 9,149,532 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Daiichi Sankyo Inc | SAVAYSA | edoxaban tosylate | TABLET;ORAL | 206316-002 | Jan 8, 2015 | RX | Yes | No | 9,149,532 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Daiichi Sankyo Inc | SAVAYSA | edoxaban tosylate | TABLET;ORAL | 206316-003 | Jan 8, 2015 | RX | Yes | Yes | 7,365,205 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Daiichi Sankyo Inc | SAVAYSA | edoxaban tosylate | TABLET;ORAL | 206316-001 | Jan 8, 2015 | RX | Yes | No | 9,149,532 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for SAVAYSA
When does loss-of-exclusivity occur for SAVAYSA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 08241982
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0809205
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 80039
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1652139
Estimated Expiration: ⤷ Get Started Free
Patent: 4324015
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 20955
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0171384
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 19377
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 40867
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 40867
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 40867
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 35990
Estimated Expiration: ⤷ Get Started Free
Patent: 800007
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 0790
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 2008129846
Estimated Expiration: ⤷ Get Started Free
Patent: 63875
Estimated Expiration: ⤷ Get Started Free
Patent: 10090168
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 140867
Estimated Expiration: ⤷ Get Started Free
Patent: 2018005
Estimated Expiration: ⤷ Get Started Free
Patent: 40867
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 9356
Patent: PHARMACEUTICAL COMPOSITION
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 1987
Patent: COMPOSICION FARMACEUTICA. (PHARMACEUTICAL COMPOSITION.)
Estimated Expiration: ⤷ Get Started Free
Patent: 09010474
Patent: COMPOSICION FARMACEUTICA. (PHARMACEUTICAL COMPOSITION.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 9725
Patent: Tablet composition having improved dissolution property
Estimated Expiration: ⤷ Get Started Free
Patent: 7109
Patent: Tablet composition having favorable dissolution property useful as an anticoagulant
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 093008
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 012500410
Patent: PHARMACEUTICAL COMPOSITION
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 40867
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 40867
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 70637
Patent: ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ (PHARMACEUTICAL COMPOSITION)
Estimated Expiration: ⤷ Get Started Free
Patent: 09139917
Patent: ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 9497
Patent: PHARMACEUTICAL COMPOSITION
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 40867
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0906182
Patent: PHARMACEUTICAL COMPOSITION
Estimated Expiration: ⤷ Get Started Free
Patent: 1005906
Patent: PHARMACEUTICAL COMPOSITION
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1424843
Estimated Expiration: ⤷ Get Started Free
Patent: 090122950
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 44803
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 09064
Estimated Expiration: ⤷ Get Started Free
Patent: 0845972
Patent: Pharmaceutical composition
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering SAVAYSA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Lithuania | PA2018005 | ⤷ Get Started Free | |
| European Patent Office | 1415992 | DERIVES DE DIAMINE (DIAMINE DERIVATIVES) | ⤷ Get Started Free |
| Australia | 2008241982 | ⤷ Get Started Free | |
| Poland | 398653 | ⤷ Get Started Free | |
| Australia | 2002346300 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SAVAYSA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1405852 | CR 2015 00052 | Denmark | ⤷ Get Started Free | PRODUCT NAME: EDOXABAN, OR A SALT THEREOF, A SOLVATE THEREOF, OR AN N-OXIDE THEREOF, IN PARTICULAR EDOXABAN TOSYLATE; REG. NO/DATE: EU/1/15/993/001-028 20150623 |
| 1405852 | 1590052-5 | Sweden | ⤷ Get Started Free | PRODUCT NAME: EDOXABAN, A SALT THEREOF, A SOLVATE THEREOF, IN PARTICULAR EDOXABAN TOSYLATE.; REG. NO/DATE: EU/1/15/993/001-028 20150623 |
| 1405852 | 2015/045 | Ireland | ⤷ Get Started Free | PRODUCT NAME: EDOXABAN, A SALT THEREOF, A SOLVATIC THEREOF, OR AN N-OXIDE THEREOF, IN PARTICULAR EDOXABAN TOSYLATE; REGISTRATION NO/DATE: EU/1/15/993/001-028 20150619 |
| 1405852 | 612 | Finland | ⤷ Get Started Free | |
| 1405852 | 51/2015 | Austria | ⤷ Get Started Free | PRODUCT NAME: EDOXABAN, EIN SALZ DAVON ODER EIN SOLVAT DAVON, INSBESONDERE EDOXABANTOSYLAT; REGISTRATION NO/DATE: EU/1/15/993/001-28 20150619 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for SAVAYSA (Eptanezumab)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
