SAPHRIS Drug Patent Profile
✉ Email this page to a colleague
When do Saphris patents expire, and what generic alternatives are available?
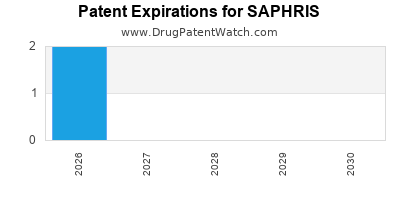
Saphris is a drug marketed by Allergan and is included in one NDA. There are two patents protecting this drug and two Paragraph IV challenges.
The generic ingredient in SAPHRIS is asenapine maleate. There are twelve drug master file entries for this compound. Seven suppliers are listed for this compound. Additional details are available on the asenapine maleate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Saphris
A generic version of SAPHRIS was approved as asenapine maleate by ALEMBIC on December 10th, 2020.
Summary for SAPHRIS
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 64 |
| Clinical Trials: | 12 |
| Patent Applications: | 87 |
| Formulation / Manufacturing: | see details |
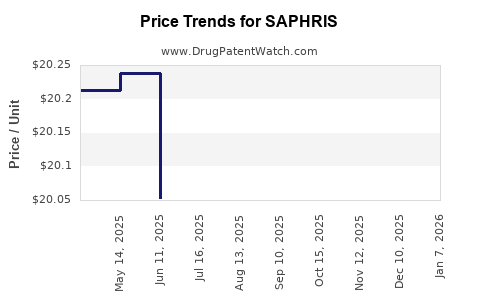
| Drug Prices: | Drug price information for SAPHRIS |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for SAPHRIS |
| What excipients (inactive ingredients) are in SAPHRIS? | SAPHRIS excipients list |
| DailyMed Link: | SAPHRIS at DailyMed |
Recent Clinical Trials for SAPHRIS
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Accutest Research Laboratories (I) Pvt. Ltd. | Phase 2/Phase 3 |
| Amneal Pharmaceuticals, LLC | Phase 2/Phase 3 |
| University of Cincinnati | Phase 2 |
Pharmacology for SAPHRIS
| Drug Class | Atypical Antipsychotic |
Anatomical Therapeutic Chemical (ATC) Classes for SAPHRIS
Paragraph IV (Patent) Challenges for SAPHRIS
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SAPHRIS | Sublingual Tablets | asenapine maleate | 2.5 mg | 022117 | 1 | 2017-07-27 |
| SAPHRIS | Sublingual Tablets | asenapine maleate | 5 mg and 10 mg | 022117 | 4 | 2013-08-13 |
US Patents and Regulatory Information for SAPHRIS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allergan | SAPHRIS | asenapine maleate | TABLET;SUBLINGUAL | 022117-003 | Mar 12, 2015 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Allergan | SAPHRIS | asenapine maleate | TABLET;SUBLINGUAL | 022117-001 | Aug 13, 2009 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Allergan | SAPHRIS | asenapine maleate | TABLET;SUBLINGUAL | 022117-003 | Mar 12, 2015 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Allergan | SAPHRIS | asenapine maleate | TABLET;SUBLINGUAL | 022117-001 | Aug 13, 2009 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SAPHRIS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Allergan | SAPHRIS | asenapine maleate | TABLET;SUBLINGUAL | 022117-001 | Aug 13, 2009 | ⤷ Sign Up | ⤷ Sign Up |
| Allergan | SAPHRIS | asenapine maleate | TABLET;SUBLINGUAL | 022117-003 | Mar 12, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| Allergan | SAPHRIS | asenapine maleate | TABLET;SUBLINGUAL | 022117-002 | Aug 13, 2009 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for SAPHRIS
See the table below for patents covering SAPHRIS around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Hungary | T76319 | ⤷ Sign Up | |
| Poland | 316080 | ⤷ Sign Up | |
| Norway | 2010024 | ⤷ Sign Up | |
| Australia | 692530 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SAPHRIS
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0746317 | 10C0056 | France | ⤷ Sign Up | PRODUCT NAME: ASENAPINE; REGISTRATION NO/DATE: EU/1/10/640/001 20100901 |
| 0746317 | SPC032/2010 | Ireland | ⤷ Sign Up | SPC032/2010: 20101210, EXPIRES: 20200228 |
| 0746317 | C300461 | Netherlands | ⤷ Sign Up | PRODUCT NAME: ASENAPINE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER HET MALEAAT; REGISTRATION NO/DATE: EU/1/10/640/001-006 20100901 |
| 0746317 | C00746317/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: ASENAPINUM; REGISTRATION NO/DATE: SWISSMEDIC 62073 20.07.2012 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


