RHOPRESSA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Rhopressa, and when can generic versions of Rhopressa launch?
Rhopressa is a drug marketed by Alcon Labs Inc and is included in one NDA. There are fourteen patents protecting this drug and one Paragraph IV challenge.
This drug has sixty-eight patent family members in fourteen countries.
The generic ingredient in RHOPRESSA is netarsudil mesylate. One supplier is listed for this compound. Additional details are available on the netarsudil mesylate profile page.
DrugPatentWatch® Generic Entry Outlook for Rhopressa
Rhopressa was eligible for patent challenges on December 18, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 14, 2034. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for RHOPRESSA
| International Patents: | 68 |
| US Patents: | 14 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 18 |
| Clinical Trials: | 5 |
| Patent Applications: | 153 |
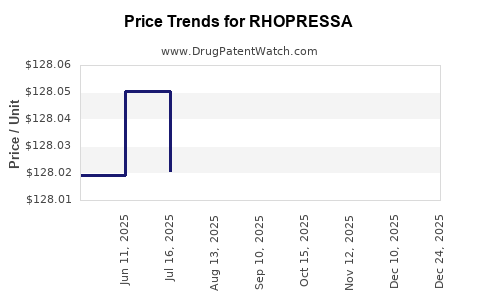
| Drug Prices: | Drug price information for RHOPRESSA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for RHOPRESSA |
| What excipients (inactive ingredients) are in RHOPRESSA? | RHOPRESSA excipients list |
| DailyMed Link: | RHOPRESSA at DailyMed |
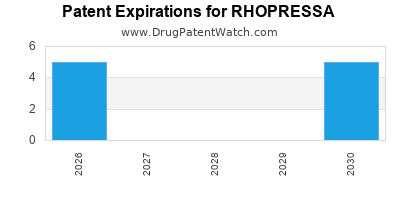
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for RHOPRESSA
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for RHOPRESSA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Wills Eye | Phase 2/Phase 3 |
| Salus University | Phase 4 |
| Price Vision Group | Phase 2/Phase 3 |
Pharmacology for RHOPRESSA
| Drug Class | Rho Kinase Inhibitor |
| Mechanism of Action | Rho Kinase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for RHOPRESSA
Paragraph IV (Patent) Challenges for RHOPRESSA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| RHOPRESSA | Ophthalmic Solution | netarsudil mesylate | 0.02% | 208254 | 2 | 2021-12-20 |
US Patents and Regulatory Information for RHOPRESSA
RHOPRESSA is protected by fourteen US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of RHOPRESSA is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting RHOPRESSA
Dual mechanism inhibitors for the treatment of disease
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: REDUCTION OF ELEVATED INTRAOCULAR PRESSURE
Beta- and gamma-amino-isoquinoline amide compounds and substituted benzamide compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: REDUCTION OF ELEVATED INTRAOCULAR PRESSURE
Combination therapy
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: REDUCTION OF ELEVATED INTRAOCULAR PRESSURE
Dual mechanism inhibitors for the treatment of disease
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: REDUCTION OF ELEVATED INTRAOCULAR PRESSURE
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: REDUCTION OF ELEVATED INTRAOCULAR PRESSURE
Beta- and gamma-amino-isoquinoline amide compounds and substituted benzamide compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: REDUCTION OF ELEVATED INTRAOCULAR PRESSURE
Dual mechanism inhibitors for the treatment of disease
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: REDUCTION OF ELEVATED INTRAOCULAR PRESSURE
Compositions for treating glaucoma or reducing intraocular pressure
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: REDUCTION OF ELEVATED INTRAOCULAR PRESSURE
Dual mechanism inhibitors for the treatment of disease
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: REDUCTION OF ELEVATED INTRAOCULAR PRESSURE
Beta- and gamma-amino-isoquinoline amide compounds and substituted benzamide compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: REDUCTION OF ELEVATED INTRAOCULAR PRESSURE
Beta- and gamma-amino-isoquinoline amide compounds and substituted benzamide compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: REDUCTION OF ELEVATED INTRAOCULAR PRESSURE
Combination therapy
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Combination therapy
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: REDUCTION OF ELEVATED INTRAOCULAR PRESSURE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcon Labs Inc | RHOPRESSA | netarsudil mesylate | SOLUTION/DROPS;OPHTHALMIC | 208254-001 | Dec 18, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Alcon Labs Inc | RHOPRESSA | netarsudil mesylate | SOLUTION/DROPS;OPHTHALMIC | 208254-001 | Dec 18, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Alcon Labs Inc | RHOPRESSA | netarsudil mesylate | SOLUTION/DROPS;OPHTHALMIC | 208254-001 | Dec 18, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Alcon Labs Inc | RHOPRESSA | netarsudil mesylate | SOLUTION/DROPS;OPHTHALMIC | 208254-001 | Dec 18, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Alcon Labs Inc | RHOPRESSA | netarsudil mesylate | SOLUTION/DROPS;OPHTHALMIC | 208254-001 | Dec 18, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Alcon Labs Inc | RHOPRESSA | netarsudil mesylate | SOLUTION/DROPS;OPHTHALMIC | 208254-001 | Dec 18, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for RHOPRESSA
When does loss-of-exclusivity occur for RHOPRESSA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 14228790
Estimated Expiration: ⤷ Sign Up
Patent: 18202965
Estimated Expiration: ⤷ Sign Up
Patent: 18202990
Estimated Expiration: ⤷ Sign Up
Patent: 20203976
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 05089
Estimated Expiration: ⤷ Sign Up
China
Patent: 5263494
Estimated Expiration: ⤷ Sign Up
Patent: 9528721
Estimated Expiration: ⤷ Sign Up
Patent: 0396085
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 11943
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 76080
Estimated Expiration: ⤷ Sign Up
Patent: 61484
Estimated Expiration: ⤷ Sign Up
Patent: 11943
Estimated Expiration: ⤷ Sign Up
Patent: 18759
Estimated Expiration: ⤷ Sign Up
Finland
Patent: 11943
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 61618
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 16515520
Estimated Expiration: ⤷ Sign Up
Patent: 19094339
Estimated Expiration: ⤷ Sign Up
Patent: 20125355
Estimated Expiration: ⤷ Sign Up
Patent: 20143163
Estimated Expiration: ⤷ Sign Up
Patent: 21046439
Estimated Expiration: ⤷ Sign Up
Patent: 23030072
Estimated Expiration: ⤷ Sign Up
Netherlands
Patent: 1101
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 11943
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 11943
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 76199
Estimated Expiration: ⤷ Sign Up
Patent: 52377
Estimated Expiration: ⤷ Sign Up
Patent: 42898
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering RHOPRESSA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Canada | 2929545 | INHIBITEURS A MECANISME DOUBLE POUR LE TRAITEMENT DE MALADIE (DUAL MECHANISM INHIBITORS FOR THE TREATMENT OF DISEASE) | ⤷ Sign Up |
| Australia | 2017248440 | DUAL MECHANISM INHIBITORS FOR THE TREATMENT OF DISEASE | ⤷ Sign Up |
| European Patent Office | 4335507 | POLYTHÉRAPIE (COMBINATION THERAPY) | ⤷ Sign Up |
| Japan | 2012525386 | ⤷ Sign Up | |
| Portugal | 3811943 | ⤷ Sign Up | |
| World Intellectual Property Organization (WIPO) | 2010126626 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for RHOPRESSA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3461484 | 21C1024 | France | ⤷ Sign Up | PRODUCT NAME: ASSOCIATION DE NETARSUDIL OU L'UN DE SES SELS ET DE LATANOPROST; REGISTRATION NO/DATE: EU/1/20/1502 20210108 |
| 3053913 | 2020/016 | Ireland | ⤷ Sign Up | PRODUCT NAME: NETARSUDIL, OR AN ENANTIOMER, DIASTEREOMER, SALT OR SOLVATE THEREOF; REGISTRATION NO/DATE: EU/1/19/1400 20191121 |
| 3461484 | SPC/GB21/033 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: A COMBINATION OF LATANOPROST AND NETARSUDIL; REGISTERED: UK EU/1/20/1502(FOR NI) 20210107; UK PLGB 16053/0034 20210107 |
| 3053913 | 2020C/510 | Belgium | ⤷ Sign Up | PRODUCT NAME: RHOKIINSA - NETARSUDIL; AUTHORISATION NUMBER AND DATE: EU/1/19/1400 20191121 |
| 3053913 | 301038 | Netherlands | ⤷ Sign Up | PRODUCT NAME: NETARSUDIL, OF EEN ZOUT OF SOLVAAAT DAARVAN, IN HET BIJZONDER NETARSUDILMESYLAAT; REGISTRATION NO/DATE: EU/1/19/1400 20191121 |
| 3461484 | 122021000036 | Germany | ⤷ Sign Up | PRODUCT NAME: LATANOPROST, ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON, UND NETARSUDIL MESYLAT; REGISTRATION NO/DATE: EU/1/20/1502 20210107 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


