RANITIDINE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Ranitidine, and what generic alternatives are available?
Ranitidine is a drug marketed by Ajanta Pharma Ltd, Appco, Aurobindo Pharma, Dr Reddys Labs Ltd, Mylan, Novitium Pharma, Sandoz, Teva, Bedford, Hikma, Mylan Labs Ltd, Zydus Pharms Usa Inc, Actavis Mid Atlantic, Amneal Pharms, Apotex Inc, Epic Pharma Llc, Lannett Co Inc, Nostrum Labs Inc, Pharm Assoc, Ranbaxy, Taro, Tolmar, Torrent, Wockhardt, Amneal Pharms Ny, Ani Pharms, Apotex, Boehringer Ingelheim, Contract Pharmacal, Dr Reddys Labs Inc, Glenmark Pharms Inc, Granules, Heritage Pharma Avet, Par Pharm, Perrigo, Perrigo R And D, Strides Pharma, Sun Pharm Inds Ltd, Thinq Pharm-cro Pvt, Vkt Pharma, Watson Labs, and Wockhardt Ltd. and is included in seventy-five NDAs.
The generic ingredient in RANITIDINE is ranitidine hydrochloride. There are forty-three drug master file entries for this compound. Thirteen suppliers are listed for this compound. Additional details are available on the ranitidine hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Ranitidine
A generic version of RANITIDINE was approved as ranitidine hydrochloride by SANDOZ on August 29th, 1997.
Summary for RANITIDINE
| US Patents: | 0 |
| Applicants: | 42 |
| NDAs: | 75 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for RANITIDINE |
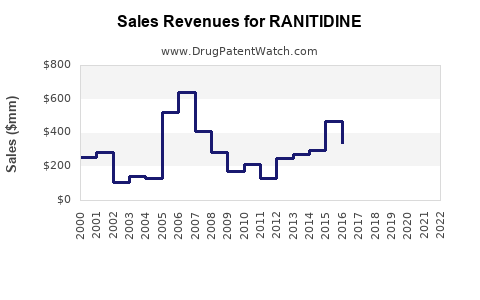
| Drug Sales Revenues: | Drug sales revenues for RANITIDINE |
| DailyMed Link: | RANITIDINE at DailyMed |

See drug prices for RANITIDINE
Recent Clinical Trials for RANITIDINE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Menoufia University | Phase 2/Phase 3 |
| Cairo University | N/A |
| University of Sao Paulo General Hospital | Phase 3 |
US Patents and Regulatory Information for RANITIDINE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Par Pharm | RANITIDINE HYDROCHLORIDE | ranitidine hydrochloride | TABLET;ORAL | 075180-001 | Jan 28, 1999 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Wockhardt | RANITIDINE HYDROCHLORIDE | ranitidine hydrochloride | TABLET;ORAL | 078884-001 | Jul 31, 2008 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Ani Pharms | RANITIDINE HYDROCHLORIDE | ranitidine hydrochloride | TABLET;ORAL | 075296-001 | Jan 14, 2000 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Ranbaxy | RANITIDINE HYDROCHLORIDE | ranitidine hydrochloride | SYRUP;ORAL | 078448-001 | Dec 13, 2007 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |


