QUTENZA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Qutenza, and what generic alternatives are available?
Qutenza is a drug marketed by Averitas and is included in one NDA. There are three patents protecting this drug.
This drug has sixty-seven patent family members in twenty-seven countries.
The generic ingredient in QUTENZA is capsaicin. There are six drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the capsaicin profile page.
DrugPatentWatch® Generic Entry Outlook for Qutenza
Qutenza was eligible for patent challenges on November 16, 2013.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 26, 2030. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for QUTENZA
| International Patents: | 67 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 150 |
| Clinical Trials: | 26 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for QUTENZA |
| What excipients (inactive ingredients) are in QUTENZA? | QUTENZA excipients list |
| DailyMed Link: | QUTENZA at DailyMed |
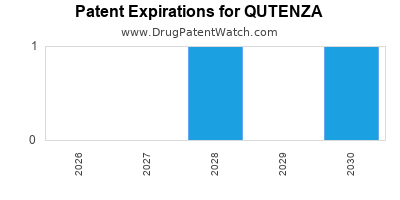

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for QUTENZA
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for QUTENZA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Leiden University Medical Center | N/A |
| Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA) | N/A |
| Averitas Pharma, Inc. | Phase 3 |
Anatomical Therapeutic Chemical (ATC) Classes for QUTENZA
US Patents and Regulatory Information for QUTENZA
QUTENZA is protected by three US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of QUTENZA is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting QUTENZA
Transdermal therapeutic system comprising an adhesive layer method for siliconizing the back layer of the system and use of said back layer
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Therapeutic patch for transdermal delivery of capsaicin
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Transdermal therapeutic system comprising an adhesive layer method for siliconizing the back layer or the system and use of said back layer
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for QUTENZA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | ⤷ Sign Up | ⤷ Sign Up |
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | ⤷ Sign Up | ⤷ Sign Up |
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | ⤷ Sign Up | ⤷ Sign Up |
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | ⤷ Sign Up | ⤷ Sign Up |
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for QUTENZA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Grunenthal GmbH | Qutenza | capsaicin | EMEA/H/C/000909 Qutenza is indicated for the treatment of peripheral neuropathic pain in adults either alone or in combination with other medicinal products for pain. |
Authorised | no | no | no | 2009-05-15 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for QUTENZA
See the table below for patents covering QUTENZA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Spain | 2288222 | ⤷ Sign Up | |
| Poland | 1791533 | ⤷ Sign Up | |
| Russian Federation | 2398567 | ТРАНСДЕРМАЛЬНАЯ ТЕРАПЕВТИЧЕСКАЯ СИСТЕМА С АДГЕЗИОННЫМ СЛОЕМ, СПОСОБ СИЛИКОНИЗАЦИИ СЛОЯ ОСНОВЫ СИСТЕМЫ И ПРИМЕНЕНИЕ СЛОЯ ОСНОВЫ (TRANSDERMAL THERAPEUTIC SYSTEM WITH ADHESION LAYER, METHOD OF SYSTEM SUBSTRATE SILICONISATION AND APPLICATION OF SUBSTRATE) | ⤷ Sign Up |
| Russian Federation | 2005134984 | ТЕРАПЕВТИЧЕСКИЙ ПЛАСТЫРЬ С ПОЛИСИЛОКСАНОВОЙ МАТРИЦЕЙ, СОДЕРЖАЩЕЙ КАПСАИЦИН | ⤷ Sign Up |
| China | 101601664 | Therapeutic patch with polysiloxane matrix comprising capsaicin and method for preparing the same | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |


