Last updated: July 28, 2025
Introduction
Prochlorperazine maleate, a dopamine antagonist classified as an antipsychotic and antiemetic agent, has carved a niche in the treatment landscape for nausea, vomiting, psychosis, and schizophrenia. Despite its longstanding presence in pharmacology, recent market dynamics and emerging trends continue to shape its financial trajectory. This analysis explores the key factors influencing current market behavior, growth prospects, competitive landscape, regulatory considerations, and the overall financial outlook for prochlorperazine maleate.
Market Overview and Therapeutic Indications
Prochlorperazine maleate was first approved in the mid-20th century and remains primarily indicated for managing severe nausea, vertigo, and psychotic disorders [1]. Its versatility lends to both hospital-based and outpatient settings. Globally, the demand for effective antiemetics sustains its usage; however, newer therapies and evolving clinical guidelines influence market share.
Market Dynamics Influencing Prochlorperazine Maleate
1. Competitive Landscape and Drug Substitution
The pharmaceutical market for antiemetics and antipsychotics is highly competitive, with the advent of serotonin receptor antagonists such as ondansetron and newer atypical antipsychotics reshaping the landscape [2]. These agents often feature improved safety profiles and increased dosing convenience, leading healthcare providers to prefer them over traditional options like prochlorperazine maleate.
2. Regulatory Environment and Patent Status
Prochlorperazine maleate's patent status varies globally; in many mature markets, it is off-patent, resulting in generic manufacturing proliferation. While generics promote affordability and wider access, they also exert downward pressure on prices and profit margins. Regulatory agencies’ approval of newer drugs further intensifies competitive pressures, sometimes leading to market erosion for traditional agents [3].
3. Clinical Practice Trends and Safety Concerns
Historically, prochlorperazine's use has been limited by its adverse effect profile, including extrapyramidal symptoms and potential for neuroleptic malignant syndrome [4]. Recent trends favor agents with fewer central nervous system side effects, impacting prescribing habits. Healthcare providers increasingly weigh the safety profiles of alternatives, especially in vulnerable populations such as pediatrics and geriatrics.
4. Rising Preference for Non-Pharmacologic Treatments
Advances in supportive care, such as improved hydration therapy, and non-pharmacological interventions are gradually reducing dependence on traditional pharmacologic antiemetics, affecting demand for drugs like prochlorperazine maleate [5].
Financial Trajectory and Market Forecast
1. Revenue Trends and Market Size
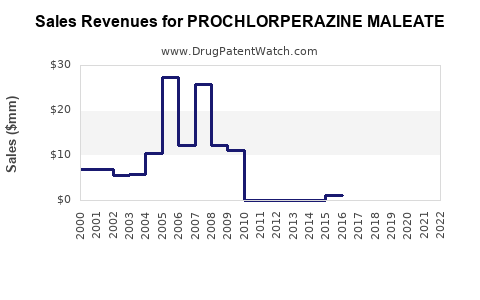
Global revenues for prochlorperazine maleate are relatively stable but modest compared to newer agents, primarily driven by established use and prescription patterns in emerging markets. In regions like Asia-Pacific and Latin America, where generic medications dominate, sales volumes remain significant despite declining per-unit pricing. Conversely, in North America and Europe, declining prescribing rates have curbed growth.
2. Impact of Patent Expiry and Generic Competition
Patent expirations have facilitated commoditization, leading to intense price competition among generic manufacturers. This trend suppresses profits but ensures broad access, especially in cost-sensitive healthcare settings. A decline in brand-name sales combined with a surge in generics can cause total revenues to plateau or decline.
3. Emerging Therapeutic Developments
Newer antiemetics with improved safety and tolerability profiles, such as olanzapine and aprepitant, show promise as substitutes, which could further hamper prochlorperazine maleate's market share. Additionally, the increasing adoption of intravenous formulations and combination therapies influences the product’s financial performance.
4. Regulatory and Geographic Market Expansion
Regulatory approvals in developing countries, where healthcare budgets favor affordable generics, sustain market presence. However, restrictions or safety concerns raised by agencies such as the FDA and EMA may limit or restrict use, affecting future revenues.
5. Future Growth Opportunities
Potential growth avenues include the development of combination formulations with improved delivery mechanisms, exploring niche indications, and expanding into emerging markets. Nevertheless, overall growth is expected to remain subdued over the next five years, with projections indicating a plateau or modest decline in global revenues.
Regulatory Considerations and Market Challenges
Regulatory bodies have issued warnings regarding prochlorperazine’s safety profile, particularly related to neurological adverse effects [6]. These warnings influence prescribing guidelines, and potential restrictions could dampen market outlooks. Market entrants focusing on safer alternatives challenge the sustained use of prochlorperazine maleate.
Conclusion
Prochlorperazine maleate’s market dynamics are characterized by mature product status, intense generic competition, evolving clinical preferences, and safety-related prescribing constraints. While it remains a mainstay in certain regions and indications, its financial trajectory faces significant headwinds from newer, safer medications and regulatory scrutiny. Future prospects hinge largely on niche applications, geographic expansion, and innovative formulation strategies, though overall growth prospects appear cautious.
Key Takeaways
- The global market for prochlorperazine maleate is declining in mature economies due to safety concerns and competition from newer agents, while emerging markets maintain steady demand due to affordability.
- Patent expirations have led to widespread generic availability, exerting downward pressure on prices and margins.
- Safety profile limitations and evolving clinical guidelines favor alternative antiemetics and antipsychotics, constraining future growth.
- Development of innovative formulations or niche indications could offer limited expansion opportunities.
- Regulatory bodies’ warnings and restrictions are critical factors that could further impact market stability.
FAQs
1. What are the primary therapeutic indications for prochlorperazine maleate?
Prochlorperazine maleate is mainly prescribed for nausea, vomiting, vertigo, and certain psychotic disorders, playing a role in both acute and chronic settings.
2. How does the patent status of prochlorperazine maleate influence its market?
Patent expirations have allowed multiple generic manufacturers to produce the drug, increasing competition, lowering prices, and reducing profitability for brand-name producers.
3. What safety concerns limit the use of prochlorperazine maleate?
Risks include extrapyramidal symptoms, neuroleptic malignant syndrome, sedation, and cardiovascular effects, leading to cautious prescribing and preference for alternatives.
4. Which markets are likely to sustain demand for prochlorperazine maleate?
Emerging markets with cost-sensitive healthcare systems continue to utilize prochlorperazine maleate, especially where affordable generics are readily available.
5. What opportunities could influence the future market for prochlorperazine maleate?
Potential growth may arise from niche indications, new formulations, or expansion into untapped geographic regions, though overall growth remains limited.
References
[1] Pharmacology textbooks and regulatory approvals (e.g., FDA, EMA documents).
[2] Market analyses from IQVIA and GlobalData.
[3] Patent and regulatory disclosures by market authorities.
[4] Clinical safety studies and prescribing guidelines.
[5] Recent literature on antiemetic treatment trends.
[6] FDA and EMA safety warnings and drug safety alerts.