PAXIL Drug Patent Profile
✉ Email this page to a colleague
When do Paxil patents expire, and when can generic versions of Paxil launch?
Paxil is a drug marketed by Apotex and is included in four NDAs.
The generic ingredient in PAXIL is paroxetine hydrochloride. There are thirty drug master file entries for this compound. Forty-three suppliers are listed for this compound. Additional details are available on the paroxetine hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Paxil
A generic version of PAXIL was approved as paroxetine hydrochloride by APOTEX on July 30th, 2003.
Summary for PAXIL
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 4 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 68 |
| Patent Applications: | 3,738 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for PAXIL |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PAXIL |
| What excipients (inactive ingredients) are in PAXIL? | PAXIL excipients list |
| DailyMed Link: | PAXIL at DailyMed |
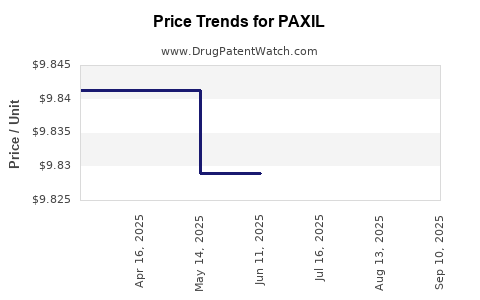
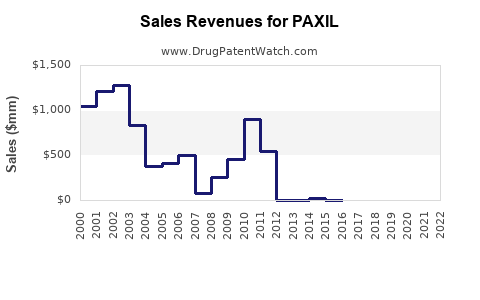
Pharmacology for PAXIL
| Drug Class | Serotonin Reuptake Inhibitor |
| Mechanism of Action | Serotonin Uptake Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for PAXIL
US Patents and Regulatory Information for PAXIL
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apotex | PAXIL | paroxetine hydrochloride | CAPSULE;ORAL | 020885-001 | Oct 9, 1998 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Apotex | PAXIL | paroxetine hydrochloride | TABLET;ORAL | 020031-002 | Dec 29, 1992 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Apotex | PAXIL | paroxetine hydrochloride | CAPSULE;ORAL | 020885-004 | Oct 9, 1998 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Apotex | PAXIL CR | paroxetine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 020936-002 | Feb 16, 1999 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Apotex | PAXIL | paroxetine hydrochloride | TABLET;ORAL | 020031-003 | Dec 29, 1992 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Apotex | PAXIL | paroxetine hydrochloride | CAPSULE;ORAL | 020885-002 | Oct 9, 1998 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Apotex | PAXIL | paroxetine hydrochloride | CAPSULE;ORAL | 020885-003 | Oct 9, 1998 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PAXIL
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Apotex | PAXIL | paroxetine hydrochloride | TABLET;ORAL | 020031-005 | Dec 29, 1992 | ⤷ Sign Up | ⤷ Sign Up |
| Apotex | PAXIL | paroxetine hydrochloride | TABLET;ORAL | 020031-003 | Dec 29, 1992 | ⤷ Sign Up | ⤷ Sign Up |
| Apotex | PAXIL | paroxetine hydrochloride | CAPSULE;ORAL | 020885-004 | Oct 9, 1998 | ⤷ Sign Up | ⤷ Sign Up |
| Apotex | PAXIL | paroxetine hydrochloride | CAPSULE;ORAL | 020885-003 | Oct 9, 1998 | ⤷ Sign Up | ⤷ Sign Up |
| Apotex | PAXIL | paroxetine hydrochloride | CAPSULE;ORAL | 020885-002 | Oct 9, 1998 | ⤷ Sign Up | ⤷ Sign Up |
| Apotex | PAXIL | paroxetine hydrochloride | TABLET;ORAL | 020031-002 | Dec 29, 1992 | ⤷ Sign Up | ⤷ Sign Up |
| Apotex | PAXIL | paroxetine hydrochloride | TABLET;ORAL | 020031-001 | Dec 29, 1992 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for PAXIL
See the table below for patents covering PAXIL around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Mexico | 9600484 | ANHIDRATO DE CLORHIDRATO DE PAROXETINA Y PROCEDIMIENTO PARA SU PREPARACION. (NOVEL COMPOUND.) | ⤷ Sign Up |
| Portugal | 102384 | ⤷ Sign Up | |
| Brazil | 9600534 | ⤷ Sign Up | |
| Hong Kong | 1037877 | ⤷ Sign Up | |
| Australia | 2002100370 | ⤷ Sign Up | |
| Czech Republic | 9103910 | ⤷ Sign Up | |
| Australia | 740561 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |


