OXYCODONE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Oxycodone, and when can generic versions of Oxycodone launch?
Oxycodone is a drug marketed by Bristol Myers Squibb, Actavis Elizabeth, Barr, Duramed Pharms Barr, Halsey, Mallinckrodt, Mutual Pharm, Vintage Pharms, Vintage Pharms Llc, Watson Labs, Abhai Llc, Mikart, Sankalp Lifecare, Specgx Llc, Alvogen, Amneal Pharms, Amneal Pharms Ny, Ascent Pharms Inc, Aurolife Pharma Llc, Chartwell, Dr Reddys Labs Sa, Elite Labs Inc, Epic Pharma Llc, Granules, Lannett Co Inc, Nesher Pharms, Novel Labs Inc, Ph Health, Rhodes Pharms, Sanaluz, Sun Pharm Inds Inc, Wes Pharma Inc, Actavis Labs Fl Inc, Ani Pharms, Sun Pharm Industries, Roxane, Avanthi Inc, Genus Lifesciences, Alkem Labs Ltd, Chartwell Molecular, Hibrow Hlthcare, Hikma, Pharm Assoc, Pharmobedient, Quagen, Vistapharm Llc, Nuvo Pharm, Strides Pharma Intl, and Barr Labs Inc. and is included in one hundred and eleven NDAs.
The generic ingredient in OXYCODONE is ibuprofen; oxycodone hydrochloride. There are sixty-four drug master file entries for this compound. Additional details are available on the ibuprofen; oxycodone hydrochloride profile page.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for OXYCODONE?
- What are the global sales for OXYCODONE?
- What is Average Wholesale Price for OXYCODONE?
Summary for OXYCODONE
| US Patents: | 0 |
| Applicants: | 49 |
| NDAs: | 111 |
| Drug Prices: | Drug price information for OXYCODONE |
| Drug Sales Revenues: | Drug sales revenues for OXYCODONE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for OXYCODONE |
| DailyMed Link: | OXYCODONE at DailyMed |
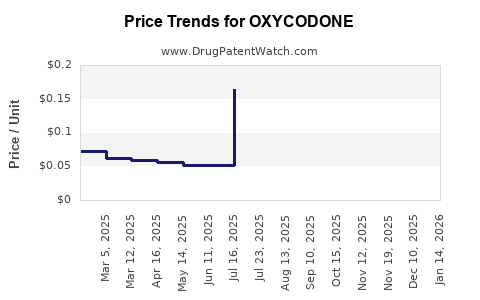
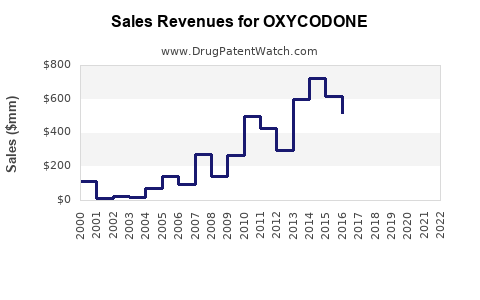
Recent Clinical Trials for OXYCODONE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Baylor College of Medicine | PHASE1 |
| Christopher D. Verrico | PHASE1 |
| University of South Alabama | PHASE4 |
Medical Subject Heading (MeSH) Categories for OXYCODONE
Anatomical Therapeutic Chemical (ATC) Classes for OXYCODONE
Paragraph IV (Patent) Challenges for OXYCODONE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| XTAMPZA ER | Extended-release Capsules | oxycodone | 9 mg, 13.5 mg, 18 mg, 27 mg and 36 mg | 208090 | 1 | 2017-11-15 |
