Last updated: July 27, 2025
Introduction
Nicotine polacrilex, also known as nicotine gum, is a form of nicotine replacement therapy (NRT) designed to aid individuals in quitting smoking. Its global market, shaped by regulatory environments, consumer health trends, and technological advancements, presents a compelling case for investors and healthcare stakeholders. This report delineates the current market dynamics, factors influencing growth, the financial trajectory, and strategic outlooks for nicotine polacrilex within the broader tobacco cessation landscape.
Market Overview
Nicotine polacrilex is one of the earliest and most widely used NRT products, alongside patches, lozenges, inhalers, and nasal sprays. The product’s efficacy in reducing withdrawal symptoms has established it as an essential component in public health efforts to lower smoking prevalence globally [1].
The global smoking cessation aids market was valued at approximately USD 3.4 billion in 2021 and is projected to grow at a compound annual growth rate (CAGR) of about 13.2% through 2030 [2]. Nicotine polacrilex holds a significant share due to its affordability, ease of use, and established safety profile.
Market Drivers
1. Rising Global Smoking Cessation Initiatives
Governments and health organizations worldwide are implementing policies to reduce smoking rates, which directly catalyzes demand for NRT products. The World Health Organization (WHO) emphasizes tobacco control, urging increased access to cessation therapies [3].
2. Increasing Awareness of Smoking-Related Health Risks
Public awareness campaigns about the risks associated with smoking—such as lung disease, cardiovascular conditions, and cancer—are encouraging smokers to seek quitting aids. This heightened consciousness boosts demand for nicotine replacement therapies, including nicotine polacrilex.
3. Regulatory Approvals and Product Accessibility
Regulatory bodies like the U.S. FDA and the European Medicines Agency have approved nicotine polacrilex for over-the-counter (OTC) use, facilitating market access [4]. Enhanced regulatory pathways and OTC status are key drivers in expanding consumer base.
4. Product Innovation and Diversification
Manufacturers are developing flavored gums and improving delivery mechanisms, increasing product appeal and compliance among diverse demographics, including youth and long-term smokers.
5. COVID-19 Pandemic Impact
The pandemic has spotlighted respiratory health, prompting increased use of cessation aids. Additionally, the pandemic-induced stressors have also elevated tobacco use in some populations, creating complex market dynamics.
Market Challenges
- Regulatory Hurdles: Stringent regulations regarding tobacco and nicotine products can restrict market entry and advertising.
- Market Saturation: Mature markets like the U.S. display high penetration rates, limiting growth scope without innovation.
- Consumer Preferences: Shift towards alternative cessation methods, such as e-cigarettes, may cannibalize traditional NRT sales.
- Pricing Pressures: Competition and regulatory controls impose downward pressure on pricing, impacting profit margins.
Financial Trajectory Analysis
Historical Performance
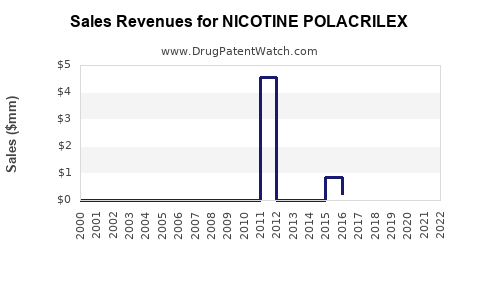
Historical data demonstrates steady revenue growth for nicotine polacrilex segments, driven by increased global smoking cessation programs. Major pharmaceutical companies and OTC manufacturers report annual sales increases in the range of 5-10% in established markets [2].
Forecasted Trends
Forecast models predict that nicotine polacrilex's market share will remain stable or grow modestly, with a projected CAGR of approximately 5-7% through 2030. Factors such as emerging markets and adult smokers seeking affordable alternatives underpin this growth trajectory [2].
Pricing and Revenue
Pricing strategies vary regionally, with premium flavored gums commanding higher prices. Volume-driven revenue growth is anticipated to counterbalance margin reductions due to price competition. The introduction of innovative formulations, such as caffeine-infused or long-lasting gums, could further augment revenue streams.
Market Segmentation
- Geographic: North America holds the largest market share, followed by Europe. Asia-Pacific is emerging as a high-growth arena owing to increasing tobacco control policies and rising awareness.
- Demographic: Adult smokers aged 25-55 represent the primary consumer base; youth prevention efforts aim to limit new initiation into nicotine use, potentially constraining future growth.
Strategic Outlook
Innovative Product Development
Investments in bioavailability, flavoring, and delivery efficacy are crucial. Combining nicotine polacrilex with digital health platforms offers personalized support, boosting cessation success rates.
Regulatory Navigation
Proactive engagement with regulatory agencies enhances market access. Harmonization of international standards can facilitate global expansion.
Market Expansion
Targeted marketing in emerging markets, leveraging unmet needs and decreasing tobacco prevalence, presents significant growth opportunities. Malaria and HIV/AIDS focus areas can catalyze health infrastructure investments conducive to NRT distribution.
Partnerships and Mergers
Collaborations with healthcare providers and incorporation into comprehensive cessation programs can increase product uptake. Mergers with or acquisitions of smaller firms specializing in flavored or technologized gums could enhance competitive positioning.
Conclusion
Nicotine polacrilex remains a vital component of tobacco cessation strategies globally. Its market is characterized by stable demand, driven by health initiatives and regulatory support, with moderate growth prospects. Companies engaging in innovation, strategic regulatory engagement, and geographic expansion are better positioned to capitalize on the evolving landscape.
Key Takeaways
- The global market for nicotine polacrilex is poised for steady growth, with a CAGR of approximately 5-7% through 2030.
- Increasing global tobacco control policies and public health campaigns sustain demand, particularly in mature markets.
- Innovation in product formulation and delivery, alongside strategic regulatory navigation, is essential for maintaining relevance and market share.
- Emerging markets offer significant future growth opportunities due to expanding health awareness and increasing tobacco cessation initiatives.
- Competitive pressures require firms to diversify, innovate, and forge strategic partnerships.
FAQs
Q1: How does nicotine polacrilex compare with other nicotine replacement therapies?
A1: Nicotine polacrilex offers quick absorption, user-controlled dosing, and affordability, making it comparable or superior to patches and lozenges in certain contexts. Its flavors and chewable format promote adherence and satisfaction.
Q2: What are the primary regulatory hurdles facing nicotine polacrilex manufacturers?
A2: Regulations focus on marketing restrictions, safety and efficacy approvals, and age restrictions. Approval pathways vary across countries, and ongoing legislative changes can impact market access.
Q3: Which regions represent the most significant growth opportunities?
A3: Asia-Pacific, Africa, and Latin America are emerging as high-growth regions due to increasing awareness, policy shifts, and large smoking populations seeking affordable cessation solutions.
Q4: How does consumer preference influence market dynamics?
A4: Preferences for flavoring, taste, and delivery influence product design and sales. The rising popularity of e-cigarettes and other alternative products also shapes consumer choices and market competition.
Q5: What future innovations could impact the nicotine polacrilex market?
A5: Innovations include biologically optimized flavors, long-lasting gums, digital adherence tools, and combination therapies integrating behavioral support with pharmacological aid.
References
[1] World Health Organization. (2020). WHO report on the global tobacco epidemic 2021.
[2] Research and Markets. (2022). Global Smoking Cessation Aids Market Forecast.
[3] WHO. (2019). Tobacco Control: Policies and Strategies.
[4] FDA. (2021). Regulatory Status of Nicotine Replacement Therapy.