NIASPAN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Niaspan, and what generic alternatives are available?
Niaspan is a drug marketed by Abbvie and is included in one NDA.
The generic ingredient in NIASPAN is niacin. There are fourteen drug master file entries for this compound. Eleven suppliers are listed for this compound. Additional details are available on the niacin profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Niaspan
A generic version of NIASPAN was approved as niacin by BARR on April 14th, 2005.
Summary for NIASPAN
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 212 |
| Clinical Trials: | 45 |
| Patent Applications: | 4,162 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for NIASPAN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for NIASPAN |
| What excipients (inactive ingredients) are in NIASPAN? | NIASPAN excipients list |
| DailyMed Link: | NIASPAN at DailyMed |
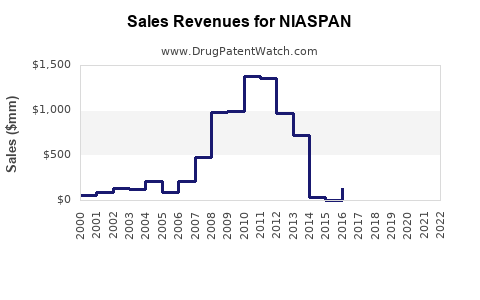
Recent Clinical Trials for NIASPAN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Pharmena North America | Phase 1 |
| Cortria Corporation | Phase 1 |
| PPD | Phase 1 |
US Patents and Regulatory Information for NIASPAN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | NIASPAN | niacin | TABLET, EXTENDED RELEASE;ORAL | 020381-004 | Jul 28, 1997 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Abbvie | NIASPAN | niacin | TABLET, EXTENDED RELEASE;ORAL | 020381-003 | Jul 28, 1997 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Abbvie | NIASPAN | niacin | TABLET, EXTENDED RELEASE;ORAL | 020381-001 | Jul 28, 1997 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Abbvie | NIASPAN | niacin | TABLET, EXTENDED RELEASE;ORAL | 020381-002 | Jul 28, 1997 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Abbvie | NIASPAN TITRATION STARTER PACK | niacin | TABLET, EXTENDED RELEASE;ORAL | 020381-005 | Jul 28, 1997 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for NIASPAN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | NIASPAN | niacin | TABLET, EXTENDED RELEASE;ORAL | 020381-004 | Jul 28, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | NIASPAN | niacin | TABLET, EXTENDED RELEASE;ORAL | 020381-002 | Jul 28, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | NIASPAN | niacin | TABLET, EXTENDED RELEASE;ORAL | 020381-003 | Jul 28, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | NIASPAN | niacin | TABLET, EXTENDED RELEASE;ORAL | 020381-001 | Jul 28, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | NIASPAN | niacin | TABLET, EXTENDED RELEASE;ORAL | 020381-003 | Jul 28, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | NIASPAN | niacin | TABLET, EXTENDED RELEASE;ORAL | 020381-004 | Jul 28, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | NIASPAN | niacin | TABLET, EXTENDED RELEASE;ORAL | 020381-003 | Jul 28, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for NIASPAN
See the table below for patents covering NIASPAN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Brazil | 9815457 | ⤷ Try a Trial | |
| Canada | 2297756 | PROCEDES DE TRAITEMENT PROPHYLACTIQUE DE SUJETS ENCLINS A L'HYPERLIPIDEMIE A L'AIDE D'UN AGENT INHIBANT LES BOUFFEES VASOMOTRICES AVANT D'ENTAMER UNE THERAPIE A BASE D'ACIDE NICOTINIQUE SOUS FORME DE DOSE QUOTIDIENNE UNIQUE DE MANIERE A REDUIRE LES BOUFFEES VASOMOTRICES PROVOQUEES PAR L'ACIDE NICOTINIQUE (METHODS OF PRETREATING HYPERLIPIDEMIC INDIVIDUALS WITH A FLUSH INHIBITING AGENT PRIOR TO THE START OF SINGLE DAILY DOSE NICOTINIC ACID THERAPY TO REDUCE FLUSHING PROVOKED BY NICOTINIC ACID) | ⤷ Try a Trial |
| Australia | 4751802 | ⤷ Try a Trial | |
| Canada | 2591710 | ⤷ Try a Trial | |
| Australia | 6454598 | ⤷ Try a Trial | |
| Australia | 8605298 | ⤷ Try a Trial | |
| Germany | 69829042 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |


