METHOTREXATE Drug Patent Profile
✉ Email this page to a colleague
When do Methotrexate patents expire, and what generic alternatives are available?
Methotrexate is a drug marketed by Accord Hlthcare, Hospira, Fresenius Kabi Usa, Abraxis Pharm, Hikma, Norbrook, Pharmachemie Usa, Epic Pharma Llc, Alembic, Amneal Pharms, Barr, Daito, Duramed Pharms Barr, Elite Labs Inc, Eugia Pharma, Lotus Pharm Co Ltd, Mylan, Strides Pharma Intl, Sun Pharm, Zydus Pharms, Eugia Pharma Speclts, Extrovis, Pharmachemie Bv, Sagent Pharms Inc, and Sandoz. and is included in forty-two NDAs.
The generic ingredient in METHOTREXATE is calcium chloride; dextrose; glutathione disulfide; magnesium chloride; potassium chloride; sodium bicarbonate; sodium chloride; sodium phosphate. There are two hundred and eighty-two drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the calcium chloride; dextrose; glutathione disulfide; magnesium chloride; potassium chloride; sodium bicarbonate; sodium chloride; sodium phosphate profile page.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for METHOTREXATE?
- What are the global sales for METHOTREXATE?
- What is Average Wholesale Price for METHOTREXATE?
Summary for METHOTREXATE
| US Patents: | 0 |
| Applicants: | 25 |
| NDAs: | 42 |
| Raw Ingredient (Bulk) Api Vendors: | 100 |
| Clinical Trials: | 2,227 |
| Patent Applications: | 2,972 |
| Drug Prices: | Drug price information for METHOTREXATE |
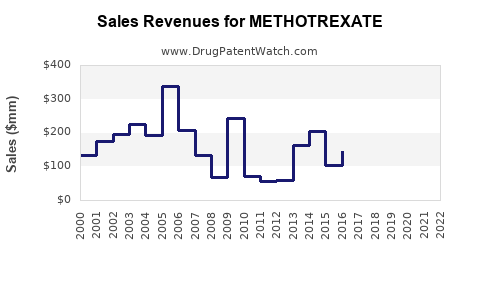
| Drug Sales Revenues: | Drug sales revenues for METHOTREXATE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for METHOTREXATE |
| What excipients (inactive ingredients) are in METHOTREXATE? | METHOTREXATE excipients list |
| DailyMed Link: | METHOTREXATE at DailyMed |
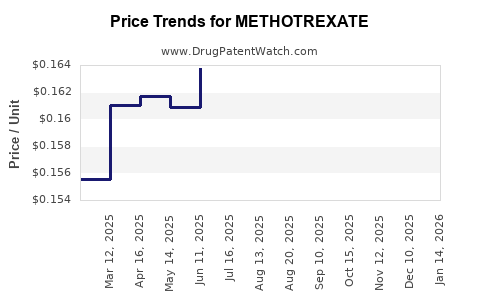
See drug prices for METHOTREXATE
Recent Clinical Trials for METHOTREXATE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Fred Hutchinson Cancer Center | PHASE2 |
| Incyte Corporation | PHASE2 |
| Dana-Farber Cancer Institute | PHASE2 |
Medical Subject Heading (MeSH) Categories for METHOTREXATE
Anatomical Therapeutic Chemical (ATC) Classes for METHOTREXATE
US Patents and Regulatory Information for METHOTREXATE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accord Hlthcare | METHOTREXATE SODIUM PRESERVATIVE FREE | methotrexate sodium | INJECTABLE;INJECTION | 040716-001 | Apr 30, 2007 | AP | RX | No | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Duramed Pharms Barr | METHOTREXATE SODIUM | methotrexate sodium | TABLET;ORAL | 040233-001 | Jun 17, 1999 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Hikma | METHOTREXATE SODIUM PRESERVATIVE FREE | methotrexate sodium | INJECTABLE;INJECTION | 040632-001 | Aug 12, 2005 | AP | RX | No | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Sagent Pharms Inc | METHOTREXATE SODIUM PRESERVATIVE FREE | methotrexate sodium | INJECTABLE;INJECTION | 203407-002 | Aug 9, 2018 | AP | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for METHOTREXATE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Nordic Group B.V. | Nordimet | methotrexate | EMEA/H/C/003983Nordimet is indicated for the treatment of:active rheumatoid arthritis in adult patients,polyarthritic forms of severe, active juvenile idiopathic arthritis (JIA), when the response to nonsteroidal anti-inflammatory drugs (NSAIDs) has been inadequate,moderate to severe plaque psoriasis in adults who are candidates for systemic therapy, and severe psoriatic arthritis in adult patients, induction of remission in moderate steroid-dependent Crohn's disease in adult patients, in combination with corticosteroids and for maintenance of remission, as monotherapy, in patients who have responded to methotrexate. | Authorised | no | no | no | 2016-08-18 | |
| Therakind (Europe) Limited | Jylamvo | methotrexate | EMEA/H/C/003756In rheumatological and dermatological diseasesActive rheumatoid arthritis in adult patients.Polyarthritic forms of active, severe juvenile idiopathic arthritis (JIA) in adolescents and children aged 3 years and over when the response to non-steroidal anti-inflammatory drugs (NSAIDs) has been inadequate.Severe, treatment-refractory, disabling psoriasis which does not respond sufficiently to other forms of treatment such as phototherapy, psoralen and ultraviolet A radiation (PUVA) therapy and retinoids, and severe psoriatic arthritis in adult patients.In oncologyMaintenance treatment of acute lymphoblastic leukaemia (ALL) in adults, adolescents and children aged 3 years and over. | Authorised | no | no | no | 2017-03-29 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
Market Dynamics and Financial Trajectory for Methotrexate: An Industry Overview
More… ↓
