ISENTRESS Drug Patent Profile
✉ Email this page to a colleague
When do Isentress patents expire, and when can generic versions of Isentress launch?
Isentress is a drug marketed by Msd Sub Merck and is included in three NDAs. There are six patents protecting this drug.
This drug has one hundred and twenty-five patent family members in forty-five countries.
The generic ingredient in ISENTRESS is raltegravir potassium. There are five drug master file entries for this compound. Nine suppliers are listed for this compound. Additional details are available on the raltegravir potassium profile page.
DrugPatentWatch® Generic Entry Outlook for Isentress
Isentress was eligible for patent challenges on October 12, 2011.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be September 11, 2029. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for ISENTRESS
| International Patents: | 125 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 3 |
| Finished Product Suppliers / Packagers: | 9 |
| Raw Ingredient (Bulk) Api Vendors: | 113 |
| Clinical Trials: | 88 |
| Patent Applications: | 4,903 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for ISENTRESS |
| What excipients (inactive ingredients) are in ISENTRESS? | ISENTRESS excipients list |
| DailyMed Link: | ISENTRESS at DailyMed |
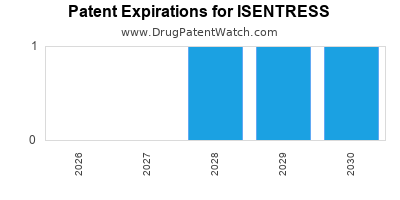
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ISENTRESS
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ISENTRESS
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Canadian Institutes of Health Research (CIHR) | Phase 2 |
| Unity Health Toronto | Phase 2 |
| St. Michael's Hospital, Toronto | Phase 2 |
Pharmacology for ISENTRESS
| Drug Class | Human Immunodeficiency Virus Integrase Strand Transfer Inhibitor |
| Mechanism of Action | HIV Integrase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for ISENTRESS
US Patents and Regulatory Information for ISENTRESS
ISENTRESS is protected by four US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ISENTRESS is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ISENTRESS
N-substituted hydroxypyrimidinone carboxamide inhibitors of HIV integrase
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Potassium salt of an HIV integrase inhibitor
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition containing an anti-nucleating agent
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF HIV INFECTION
Pharmaceutical formulation containing a release rate controlling composition
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF HIV INFECTION
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Msd Sub Merck | ISENTRESS | raltegravir potassium | POWDER;ORAL | 205786-001 | Dec 20, 2013 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Msd Sub Merck | ISENTRESS | raltegravir potassium | TABLET, CHEWABLE;ORAL | 203045-002 | Dec 21, 2011 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Msd Sub Merck | ISENTRESS | raltegravir potassium | TABLET, CHEWABLE;ORAL | 203045-001 | Dec 21, 2011 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ISENTRESS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Msd Sub Merck | ISENTRESS | raltegravir potassium | TABLET;ORAL | 022145-001 | Oct 12, 2007 | ⤷ Sign Up | ⤷ Sign Up |
| Msd Sub Merck | ISENTRESS | raltegravir potassium | POWDER;ORAL | 205786-001 | Dec 20, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| Msd Sub Merck | ISENTRESS | raltegravir potassium | POWDER;ORAL | 205786-001 | Dec 20, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ISENTRESS
When does loss-of-exclusivity occur for ISENTRESS?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2034
Estimated Expiration: ⤷ Sign Up
Patent: 1429
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 05311671
Estimated Expiration: ⤷ Sign Up
Patent: 10313571
Estimated Expiration: ⤷ Sign Up
Austria
Patent: 18844
Estimated Expiration: ⤷ Sign Up
Patent: 34645
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0518760
Estimated Expiration: ⤷ Sign Up
Patent: 2012009857
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 88398
Estimated Expiration: ⤷ Sign Up
Patent: 77937
Estimated Expiration: ⤷ Sign Up
China
Patent: 1068793
Estimated Expiration: ⤷ Sign Up
Patent: 2655752
Estimated Expiration: ⤷ Sign Up
Patent: 6074411
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 31485
Estimated Expiration: ⤷ Sign Up
Costa Rica
Patent: 46
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0120066
Estimated Expiration: ⤷ Sign Up
Patent: 0211826
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 12859
Estimated Expiration: ⤷ Sign Up
Patent: 24914
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 19700
Estimated Expiration: ⤷ Sign Up
Patent: 93312
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 2418
Estimated Expiration: ⤷ Sign Up
Patent: 0701204
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 19683
Estimated Expiration: ⤷ Sign Up
Patent: 19700
Estimated Expiration: ⤷ Sign Up
Patent: 93312
Estimated Expiration: ⤷ Sign Up
Patent: 70702
Estimated Expiration: ⤷ Sign Up
Georgia, Republic of
Patent: 0105086
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 15011
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 57248
Estimated Expiration: ⤷ Sign Up
India
Patent: 77DEN2012
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 3614
Estimated Expiration: ⤷ Sign Up
Patent: 9369
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 05956
Estimated Expiration: ⤷ Sign Up
Patent: 22639
Estimated Expiration: ⤷ Sign Up
Patent: 08521933
Estimated Expiration: ⤷ Sign Up
Patent: 13508395
Estimated Expiration: ⤷ Sign Up
Patent: 16034962
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 93312
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 4320
Estimated Expiration: ⤷ Sign Up
Patent: 2494
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 5227
Estimated Expiration: ⤷ Sign Up
Patent: 07006639
Estimated Expiration: ⤷ Sign Up
Patent: 12004903
Estimated Expiration: ⤷ Sign Up
Montenegro
Patent: 985
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 120
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 5376
Estimated Expiration: ⤷ Sign Up
Patent: 0331
Estimated Expiration: ⤷ Sign Up
Nicaragua
Patent: 0700138
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 8784
Estimated Expiration: ⤷ Sign Up
Patent: 073404
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 061148
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 19700
Estimated Expiration: ⤷ Sign Up
Patent: 93312
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 19700
Estimated Expiration: ⤷ Sign Up
Patent: 93312
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 02865
Estimated Expiration: ⤷ Sign Up
Patent: 12121857
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 197
Estimated Expiration: ⤷ Sign Up
Patent: 600
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 19700
Estimated Expiration: ⤷ Sign Up
Patent: 93312
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 0704130
Estimated Expiration: ⤷ Sign Up
Patent: 1203012
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1350420
Estimated Expiration: ⤷ Sign Up
Patent: 1835893
Estimated Expiration: ⤷ Sign Up
Patent: 070089990
Estimated Expiration: ⤷ Sign Up
Patent: 120102063
Estimated Expiration: ⤷ Sign Up
Patent: 130122031
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 70136
Estimated Expiration: ⤷ Sign Up
Patent: 75788
Estimated Expiration: ⤷ Sign Up
Patent: 98348
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 44463
Estimated Expiration: ⤷ Sign Up
Patent: 0631944
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 07215
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 884
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ISENTRESS around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2013063999 | PHARMACEUTICAL COMPOSITION CONTAINING ANTI-NUCLEATING AGENT | ⤷ Sign Up |
| Israel | 161337 | N-SUBSTITUTED HYDROXYPYRIMIDINONE CARBOXAMIDE INHIBITORS OF HIV INTEGRASE | ⤷ Sign Up |
| South Korea | 100862879 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ISENTRESS
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1441735 | 319 | Finland | ⤷ Sign Up | |
| 1441735 | PA2008 007, C1441735 | Lithuania | ⤷ Sign Up | PRODUCT NAME: RALTEGRAVIRUM; REGISTRATION NO/DATE: EU/1/07/436/001-002 20071220 |
| 1441735 | C200800017 | Spain | ⤷ Sign Up | PRODUCT NAME: RALTEGRAVIR; NATIONAL AUTHORISATION NUMBER: UE/1/07/436/001-002; DATE OF AUTHORISATION: 20071220; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): UE/1/07/436/001-002; DATE OF FIRST AUTHORISATION IN EEA: 20071220 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


