ISENTRESS Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Isentress, and when can generic versions of Isentress launch?
Isentress is a drug marketed by Msd Sub Merck and is included in three NDAs. There are five patents protecting this drug.
This drug has seventy-six patent family members in thirty-seven countries.
The generic ingredient in ISENTRESS is raltegravir potassium. There are five drug master file entries for this compound. Five suppliers are listed for this compound. Additional details are available on the raltegravir potassium profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Isentress
A generic version of ISENTRESS was approved as raltegravir potassium by LUPIN LTD on May 6th, 2025.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ISENTRESS?
- What are the global sales for ISENTRESS?
- What is Average Wholesale Price for ISENTRESS?
Summary for ISENTRESS
| International Patents: | 76 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 3 |
| Finished Product Suppliers / Packagers: | 5 |
| Raw Ingredient (Bulk) Api Vendors: | 76 |
| Clinical Trials: | 88 |
| Patent Applications: | 350 |
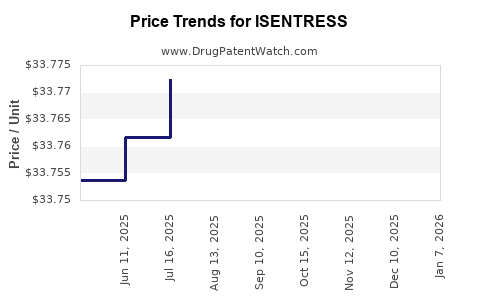
| Drug Prices: | Drug price information for ISENTRESS |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ISENTRESS |
| What excipients (inactive ingredients) are in ISENTRESS? | ISENTRESS excipients list |
| DailyMed Link: | ISENTRESS at DailyMed |
Recent Clinical Trials for ISENTRESS
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Unity Health Toronto | Phase 2 |
| St. Michael's Hospital, Toronto | Phase 2 |
| Canadian Institutes of Health Research (CIHR) | Phase 2 |
Pharmacology for ISENTRESS
| Drug Class | Human Immunodeficiency Virus Integrase Strand Transfer Inhibitor |
| Mechanism of Action | HIV Integrase Inhibitors |
US Patents and Regulatory Information for ISENTRESS
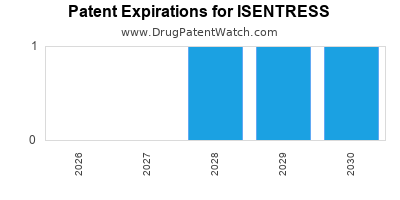
ISENTRESS is protected by three US patents.
Expired US Patents for ISENTRESS
International Patents for ISENTRESS
When does loss-of-exclusivity occur for ISENTRESS?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 10313571
Patent: Solid pharmaceutical compositions containing an integrase inhibitor
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2012009857
Patent: composições farmacêuticas sólidas contendo um inibidor de integrase
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 77937
Patent: COMPOSITIONS PHARMACEUTIQUES SOLIDES CONTENANT UN INHIBITEUR D'INTEGRASE (SOLID PHARMACEUTICAL COMPOSITIONS CONTAINING AN INTEGRASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2655752
Patent: Solid pharmaceutical compositions containing an integrase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 6074411
Patent: 包含整合酶抑制剂的固体药物组合物 (SOLID PHARMACEUTICAL COMPOSITIONS CONTAINING AN INTEGRASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 31485
Patent: COMPOSICIONES FARMACÉUTICAS SÓLIDAS QUE CONTIENEN UN INHIBIDOR DE LA INTEGRASA
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0211826
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 24914
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 93312
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 93312
Patent: COMPOSITIONS PHARMACEUTIQUES SOLIDES CONTENANT UN INHIBITEUR D'INTÉGRASE (SOLID PHARMACEUTICAL COMPOSITIONS CONTAINING AN INTEGRASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 70702
Patent: COMPOSITIONS PHARMACEUTIQUES SOLIDES CONTENANT UN INHIBITEUR D'INTÉGRASE (SOLID PHARMACEUTICAL COMPOSITIONS CONTAINING AN INTEGRASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 57248
Estimated Expiration: ⤷ Get Started Free
India
Patent: 77DEN2012
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 9369
Patent: תכשירים רוקחיים מוצקים המכילים מעכב אינטגראז (Solid pharmaceutical compositions containing an integrase inhibitor)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 22639
Estimated Expiration: ⤷ Get Started Free
Patent: 13508395
Estimated Expiration: ⤷ Get Started Free
Patent: 16034962
Patent: インテグラーゼ阻害剤を含有する固形医薬組成物 (SOLID PHARMACEUTICAL COMPOSITIONS CONTAINING INTEGRASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 93312
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 2494
Patent: SOLID PHARMACEUTICAL COMPOSITIONS CONTAINING AN INTEGRASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 5227
Patent: COMPOSICIONES FARMACEUTICAS SOLIDAS QUE CONTIENEN UN INHIBIDOR DE LA INTEGRASA. (SOLID PHARMACEUTICAL COMPOSITIONS CONTAINING AN INTEGRASE INHIBITOR.)
Estimated Expiration: ⤷ Get Started Free
Patent: 12004903
Patent: COMPOSICIONES FARMACEUTICAS SOLIDAS QUE CONTIENEN UN HINIBIDOR DE LA INTEGRASA. (SOLID PHARMACEUTICAL COMPOSITIONS CONTAINING AN INTEGRASE INHIBITOR.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 0331
Patent: Solid pharmaceutical compositions containing an integrase inhibitor
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 93312
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 93312
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 02865
Patent: ТВЕРДЫЕ ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ, СОДЕРЖАЩИЕ ИНГИБИТОР ИНТЕГРАЗЫ (SOLID PHARMACEUTICAL COMPOSITIONS CONTAINING INTEGRASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 12121857
Patent: ТВЕРДЫЕ ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ, СОДЕРЖАЩИЕ ИНГИБИТОР ИНТЕГРАЗЫ
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 600
Patent: ČVRSTE FARMACEUTSKE KOMPOZICIJE KOJE SADRŽE INHIBITOR INTEGRAZE (SOLID PHARMACEUTICAL COMPOSITIONS CONTAINING AN INTEGRASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 93312
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1203012
Patent: SOLID PHARMACEUTICAL COMPOSITIONS CONTAINING AN INTERGRASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1835893
Estimated Expiration: ⤷ Get Started Free
Patent: 120102063
Patent: SOLID PHARMACEUTICAL COMPOSITIONS CONTAINING AN INTEGRASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 98348
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ISENTRESS around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Russian Federation | 2012121857 | ТВЕРДЫЕ ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ, СОДЕРЖАЩИЕ ИНГИБИТОР ИНТЕГРАЗЫ | ⤷ Get Started Free |
| Georgia, Republic of | P20063848 | N-SUBSTITUTED HYDROXYPYRIMI-DINONE CARBOXAMIDE INHIBITORS OF HIV INTEGRASE | ⤷ Get Started Free |
| Portugal | 1819700 | ⤷ Get Started Free | |
| Brazil | 112012009857 | composições farmacêuticas sólidas contendo um inibidor de integrase | ⤷ Get Started Free |
| Tunisia | SN07215 | POTASSIUM SALT OF AN HIV INTEGRASE INHIBITOR | ⤷ Get Started Free |
| Montenegro | 01985 | KALIJUMOVA SO INHIBITORA HIV INTEGRAZE (POTASSIUM SALT OF AN HIV INTEGRASE INHIBITOR) | ⤷ Get Started Free |
| Norway | 2008007 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ISENTRESS
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1441735 | 30/2008 | Austria | ⤷ Get Started Free | PRODUCT NAME: RALTEGRAVIR ODER DESSEN PHARMAZEUTISCH ANNEHMBAREN SALZE, INSBESONDERE DAS KALIUMSALZ; REGISTRATION NO/DATE: EU/1/07/436 (MITTEILUNG) 20071220 |
| 1441735 | C20080001 00016 | Estonia | ⤷ Get Started Free | PRODUCT NAME: ISENTRESS; REG NO/DATE: 20.12.2007 C(2007)6801 |
| 1441735 | 2008/010 | Ireland | ⤷ Get Started Free | PRODUCT NAME: RALTEGRAVIR OR A PHARMECEUTICALLY ACCEPTABLE SALT THEREOF, ESPECIALLY THE POTASSIUM SALT; NAT AUTHORISTION NO/DATE: EU/1/07/436/001-002 20071220; |
| 1441735 | C200800017 | Spain | ⤷ Get Started Free | PRODUCT NAME: RALTEGRAVIR; NATIONAL AUTHORISATION NUMBER: UE/1/07/436/001-002; DATE OF AUTHORISATION: 20071220; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): UE/1/07/436/001-002; DATE OF FIRST AUTHORISATION IN EEA: 20071220 |
| 1441735 | 20221021 | Netherlands | ⤷ Get Started Free | 20221021, EXPIRES: 20221219 |
| 1441735 | PA2008 007, C1441735 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: RALTEGRAVIRUM; REGISTRATION NO/DATE: EU/1/07/436/001-002 20071220 |
| 1441735 | C01441735/01 | Switzerland | ⤷ Get Started Free | FORMER OWNER: ISTITUTO DI RICERCHE DI BIOLOGIA MOLECOLARE P. ANGELETTI S.P.A., IT |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for ISENTRESS (Raltegravir)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
