Last updated: July 28, 2025
Introduction
Ibandronate sodium, a nitrogen-containing bisphosphonate, serves as a pivotal agent in the treatment and prevention of osteoporosis, a condition characterized by decreased bone density and increased fracture risk. Its unique pharmacological properties have positioned it within the lucrative bisphosphonate segment, influencing both market dynamics and financial trajectories faced by pharmaceutical companies. This article explores the evolving landscape of ibandronate sodium, examining current market drivers, competitive factors, regulatory influences, and future financial outlooks that shape its commercial trajectory.
Pharmacological Profile and Therapeutic Significance
Ibandronate sodium stabilizes bone mass by inhibiting osteoclast-mediated bone resorption, making it an essential therapy for postmenopausal osteoporosis. Its administration, either as an oral tablet or intravenous injection, offers flexibility and improved patient compliance. The drug's efficacy in reducing vertebral fractures has established its clinical importance, especially among high-risk populations.
Market Drivers
1. Growing Osteoporosis Prevalence
Rising global aging populations directly contribute to an expanding demographic vulnerable to osteoporosis. The World Health Organization projects that the number of osteoporotic fractures will exceed 4.5 million annually in Europe alone by 2040, a trend that accelerates demand for bone-related therapies, including ibandronate sodium [1].
2. Advancements in Drug Formulations and Delivery
Innovations, such as monthly oral tablets and monthly IV dosing, have enhanced dosing convenience, improving adherence. These formulations, approved by regulatory bodies like the FDA and EMA, have widened the drug's appeal, especially among elderly populations who struggle with complex regimens.
3. Increasing Awareness and Screening Programs
Enhanced screening strategies and increased awareness have led to earlier diagnosis and initiation of osteoporosis treatments. This proactive approach amplifies demand for first-line agents like ibandronate sodium.
4. Rising Healthcare Expenditure
Growing healthcare budgets across developed nations facilitate broader access to osteoporosis therapies, including branded formulations, thus bolstering market growth.
Competitive Landscape
1. Patent Status and Market Exclusivity
Currently, ibandronate sodium’s patent life varies by region, with many markets witnessing patent expirations that facilitate generic entry. Biogen’s Boniva was among the first marketed in this class, but recent patent lapses have opened avenues for generics, intensifying price competition.
2. Key Market Players
- Roche/Genentech (original licensee of Boniva): Pioneered clinical development and marketing strategies.
- Teva Pharmaceuticals and Mylan: Leading generic manufacturers increasing market penetration.
- Other Players: Several regional manufacturers are developing biosimilar and generic versions, escalating competitive pressure.
3. Market Share Trends
Branded versions historically held dominance; however, post-patent expirations have radically shifted market share towards generics, exerting downward pressure on drug prices and profit margins.
4. Competition from Alternative Therapies
Newer osteoporosis treatments, such as denosumab (a monoclonal antibody) and anabolic agents like teriparatide, challenge ibandronate sodium’s market share, especially in patients with contraindications or intolerance.
Regulatory and Market Challenges
1. Safety and Tolerability Concerns
Reports of adverse effects, including osteonecrosis of the jaw and atypical femoral fractures, have prompted regulatory agencies to update warnings, potentially impacting prescribing patterns and sales.
2. Reimbursement Policies
Variations in reimbursement across markets influence patient access and adherence. Tightened reimbursement policies can hamper sales growth, especially in price-sensitive markets.
3. Market Saturation and Regional Variance
Developed economies exhibit high penetration; emerging markets still present untapped opportunities, albeit with challenges related to healthcare infrastructure and awareness.
Financial Trajectory and Revenue Outlook
1. Impact of Patent Expirations
Patent expirations in key markets, such as the United States and Europe, have shifted revenues from branded to generic ibandronate sodium. While this reduces average selling prices (ASPs), volume increases maintain overall revenue levels temporarily. For example, Roche reported a decline in Boniva sales post-patent expiry but continued to benefit from volume-driven growth in generics.
2. Pricing Dynamics
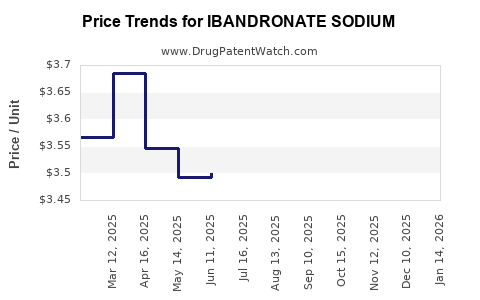
The proliferation of generics has resulted in significant price erosion—estimated reductions of up to 80% since patent expiration—reflecting a highly price-sensitive market. Companies actively seeking geographic or niche markets aim to offset losses.
3. R&D and Pipeline Opportunities
Innovative formulations, such as long-acting injectables with improved dosing intervals or combination therapies, could rejuvenate growth. Additionally, biosimilar development presents new revenue streams, though regulatory hurdles persist.
4. Market Penetration in Emerging Economies
Emerging markets like China and India demonstrate promising growth trajectories owing to rising osteoporosis awareness and increasing healthcare infrastructure investments. Price adjustments aligned with local purchasing power remain critical for success.
5. Impact of New Therapeutic Alternatives
The advent of newer agents with favorable safety profiles and convenience may limit ibandronate sodium’s market share expansion. Nonetheless, cost-effectiveness and familiarity make it a continued staple in certain segments.
Future Outlook and Strategic Considerations
Given the ongoing patent expiry trends and the emergence of competing therapies, the financial trajectory of ibandronate sodium is poised for transition. Companies investing in pipeline innovations—such as ultra-long-acting formulations or novel delivery mechanisms—may mitigate declining revenues and stimulate growth within niche markets. Furthermore, strategic alliances and licensing agreements serve as crucial tools to expand geographical reach and adapt to market shifts.
Key Market Trends Summary
- Aging populations globally underpin sustained demand, although growth rates are tapering in mature markets.
- Patent expirations have catalyzed generic proliferation, diminishing revenue per unit but expanding market volume.
- Innovation in formulations can revitalize product relevance and capture unmet needs.
- Competitive pressures from newer therapies necessitate differentiation strategies.
- Regional disparities highlight opportunities for tailored market entry and growth strategies, especially in emerging economies.
Key Takeaways
- Market maturation driven by patent expiries has shifted revenue models from proprietary to generic sales, necessitating strategic diversification.
- Pricing strategies must adapt to increasing generic competition and price sensitivity, especially in cost-conscious regions.
- Innovation pipeline—including long-acting formulations and combination therapies—presents avenues to sustain growth.
- Regulatory and safety updates continue to influence prescribing behaviors and market access.
- Emerging markets offer high-growth opportunities, contingent on tailored pricing, distribution, and educational initiatives.
FAQs
1. How has patent expiration affected the revenue of ibandronate sodium?
Patent expiration has led to a surge in generic options, significantly reducing the drug’s price and profit margins for branded companies. While overall sales volume has increased, revenues from branded formulations have declined, prompting firms to seek alternative revenue streams such as pipeline innovations or market expansion.
2. What are the primary competitors to ibandronate sodium in osteoporosis treatment?
Primary competitors include other bisphosphonates like alendronate and zoledronic acid, as well as newer therapies like denosumab (a RANK ligand inhibitor) and anabolic agents like teriparatide. These alternatives often boast improved safety profiles or dosing regimens, challenging ibandronate's market position.
3. Which regions present the most promising growth prospects for ibandronate sodium?
Emerging markets, notably China, India, and parts of Latin America, exhibit significant growth potential due to increasing osteoporosis prevalence, expanding healthcare infrastructure, and evolving treatment awareness.
4. How do safety concerns influence the market dynamics of ibandronate sodium?
Safety concerns—such as osteonecrosis of the jaw and atypical fractures—prompt regulatory updates and cautionary labeling, potentially limiting prescriptions. Ongoing safety monitoring and improved formulations could mitigate these issues and support market stability.
5. What strategic moves should pharmaceutical companies consider to maximize returns from ibandronate sodium?
Companies should focus on developing long-acting formulations, exploring combination therapies, expanding geographic reach into growth markets, and investing in biosimilar development to offset revenue declines and maintain competitive advantage.
References
- World Health Organization. Osteoporosis Fact Sheet. 2021.