HORIZANT Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Horizant, and what generic alternatives are available?
Horizant is a drug marketed by Azurity and is included in one NDA. There are five patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and forty-six patent family members in twenty-six countries.
The generic ingredient in HORIZANT is gabapentin enacarbil. There are twenty-nine drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the gabapentin enacarbil profile page.
DrugPatentWatch® Generic Entry Outlook for Horizant
Horizant was eligible for patent challenges on April 6, 2015.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 10, 2029. This may change due to patent challenges or generic licensing.
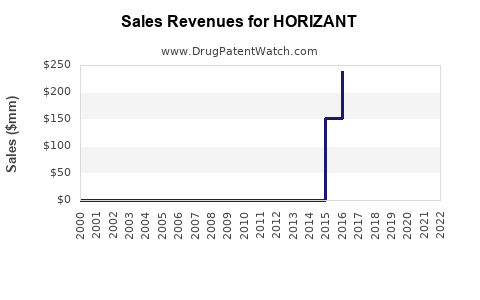
Annual sales in 2021 were $171mm indicating the motivation for generic entry (peak sales were $535mm in 2018).
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
Summary for HORIZANT
| International Patents: | 146 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 61 |
| Clinical Trials: | 14 |
| Patent Applications: | 253 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for HORIZANT |
| Drug Sales Revenues: | Drug sales revenues for HORIZANT |
| What excipients (inactive ingredients) are in HORIZANT? | HORIZANT excipients list |
| DailyMed Link: | HORIZANT at DailyMed |
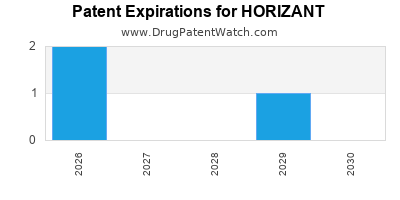
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for HORIZANT
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for HORIZANT
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Massachusetts General Hospital | Phase 4 |
| University of California, Davis | Phase 3 |
| National Institute on Aging (NIA) | Phase 4 |
Pharmacology for HORIZANT
| Physiological Effect | Decreased Central Nervous System Disorganized Electrical Activity |
Anatomical Therapeutic Chemical (ATC) Classes for HORIZANT
Paragraph IV (Patent) Challenges for HORIZANT
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| HORIZANT | Extended-release Tablets | gabapentin enacarbil | 300 mg and 600 mg | 022399 | 1 | 2019-04-29 |
US Patents and Regulatory Information for HORIZANT
HORIZANT is protected by seven US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of HORIZANT is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting HORIZANT
Prodrugs of GABA analogs, compositions and uses thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Crystalline form of .gamma.-aminobutyric acid analog
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Treating or preventing restless legs syndrome using prodrugs of GABA analogs
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF MODERATE-TO-SEVERE PRIMARY RESTLESS LEG SYNDROME IN ADULTS
Crystalline form of .gamma.-aminobutyric acid analog
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF MODERATE-TO-SEVERE PRIMARY RESTLESS LEG SYNDROME IN ADULTS
Crystalline form of .gamma.-aminobutyric acid analog
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: MANAGEMENT OF POSTHERPETIC NEURALGIA (PHN) IN ADULTS
GABA analog prodrug sustained release oral dosage forms
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF MODERATE-TO-SEVERE PRIMARY RESTLESS LEG SYNDROME IN ADULTS
GABA analog prodrug sustained release oral dosage forms
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: MANAGEMENT OF POSTHERPETIC NEURALGIA (PHN) IN ADULTS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azurity | HORIZANT | gabapentin enacarbil | TABLET, EXTENDED RELEASE;ORAL | 022399-002 | Dec 13, 2011 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Azurity | HORIZANT | gabapentin enacarbil | TABLET, EXTENDED RELEASE;ORAL | 022399-002 | Dec 13, 2011 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Azurity | HORIZANT | gabapentin enacarbil | TABLET, EXTENDED RELEASE;ORAL | 022399-002 | Dec 13, 2011 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Azurity | HORIZANT | gabapentin enacarbil | TABLET, EXTENDED RELEASE;ORAL | 022399-001 | Apr 6, 2011 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for HORIZANT
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Azurity | HORIZANT | gabapentin enacarbil | TABLET, EXTENDED RELEASE;ORAL | 022399-002 | Dec 13, 2011 | ⤷ Sign Up | ⤷ Sign Up |
| Azurity | HORIZANT | gabapentin enacarbil | TABLET, EXTENDED RELEASE;ORAL | 022399-001 | Apr 6, 2011 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for HORIZANT
When does loss-of-exclusivity occur for HORIZANT?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 05301970
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0517227
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 84338
Estimated Expiration: ⤷ Sign Up
China
Patent: 1068538
Estimated Expiration: ⤷ Sign Up
Patent: 2429882
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 11986
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 04798
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 56095
Estimated Expiration: ⤷ Sign Up
Patent: 08518971
Estimated Expiration: ⤷ Sign Up
Patent: 13107900
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 07005306
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 4737
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 8688
Estimated Expiration: ⤷ Sign Up
Patent: 072767
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 40112
Estimated Expiration: ⤷ Sign Up
Patent: 07120570
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 0703571
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1228399
Estimated Expiration: ⤷ Sign Up
Patent: 070074604
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 77938
Estimated Expiration: ⤷ Sign Up
Patent: 0630086
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering HORIZANT around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Russian Federation | 2006112549 | ЛЕЧЕНИЕ ИЛИ ПРЕДУПРЕЖДЕНИЕ СИНДРОМА БЕСПОКОЙНЫХ НОГ С ИСПОЛЬЗОВАНИЕМ ПРОЛЕКАРСТВ АНАЛОГОВ ГАМК | ⤷ Sign Up |
| China | 101434572 | Prodrugs of GABA analogs, compositions and uses thereof | ⤷ Sign Up |
| Israel | 202603 | הרכב רוקחי המכיל 1 } ]איזובוטאנוילאוקסיאתוקסי) קרבוניל ]אמינומתיל{-1-ציקלוהקסן חומצה אצטית גבישית, והרכב רוקחי מקובל והשימוש שלו להכנת תרופה (Pharmaceutical composition comprising crystalline 1{[(a-isobutanoyloxyethoxy)carbonyl]aminomethyl}-1-cyclohexane acetic acid and a pharmaceutically acceptable vehicle and use thereof for preparation of a medicament) | ⤷ Sign Up |
| Hungary | P0400167 | GABA-analógok elõanyagai, ezek készítményei és alkalmazásai (PRODRUGS OF GABA ANALOGS, COMPOSITIONS AND USES THEREOF) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


