Last updated: July 27, 2025
Introduction
Flecainide acetate is a prominent antiarrhythmic medication primarily used to treat atrial fibrillation, atrial flutter, and ventricular arrhythmias. As a Class IC agent, it is renowned for its efficacy in managing complex arrhythmias resistant to other therapeutic options. Over recent years, market trends, regulatory shifts, and evolving cardiovascular treatment paradigms have significantly influenced the drug’s market dynamics and financial outlook.
Market Overview
Global Demand and Therapeutic Use
Flecainide acetate’s worldwide application is driven by the rising prevalence of atrial fibrillation (AF), which affects an estimated 33 million individuals globally, a figure projected to rise in tandem with aging populations and increasing cardiovascular risk factors [1]. The drug holds a substantial share in second-line antiarrhythmic therapies, especially in developed markets like North America and Europe, where arrhythmia management is sophisticated and protocol-driven.
Key Market Segments and Sources
The primary sources of revenue for flecainide acetate stem from prescription sales in hospitals, specialty clinics, and outpatient settings. Generic versions have entered multiple markets, impacting pricing strategies and revenue streams for branded formulations.
Market Dynamics
1. Regulatory Environment and Patent Landscape
Flecainide acetate was first approved in the United States in the late 1980s, with subsequent approvals worldwide. Patent expirations for the original formulations have led to a surge in generic entrants, exerting downward pressure on prices [2]. Regulatory agencies’ emphasis on safety surveillance and post-marketing studies has also influenced market stability, especially after concerns relating to proarrhythmic risk surfaced in certain patient subsets.
2. Competitive Landscape
The antiarrhythmic drug market features several classes: sodium channel blockers, beta-blockers, and other Class IC agents. Flecainide’s main competitors include propafenone, amiodarone, and sotalol. Generic competition has significantly diversified options, with generics capturing a larger share, thereby affecting branded drug revenues.
3. Technological and Clinical Developments
Advances in catheter ablation and wearable cardiac monitoring devices have transformed arrhythmia management. These technologies sometimes supplant pharmacologic therapy, impacting the demand for antiarrhythmic drugs. Nevertheless, flecainide acetate remains relevant for patients unsuitable for invasive procedures or where pharmacologic control is essential.
4. Market Penetration and Regional Variations
North America accounts for a substantial portion of the market, driven by robust healthcare infrastructure, high disease prevalence, and expert cardiology centers. Europe follows, with growing adoption in Eastern European countries. Asian markets exhibit expanding demand owing to increasing cardiovascular disease burden and improved healthcare access. However, regulatory approvals vary, influencing regional market size.
Financial Trajectory
1. Market Revenue Trends
The pharmaceutical market research indicates that, prior to the patent expirations around the early 2000s, branded flecainide formulations contributed significantly to pharmaceutical revenues, estimated at over $200 million annually in the U.S. alone [3]. Post-generic entry, revenues declined sharply, with current estimates placing global sales at approximately $50 million to $75 million annually, predominantly from branded sales in niche markets.
2. Impact of Patent Expiry and Generics
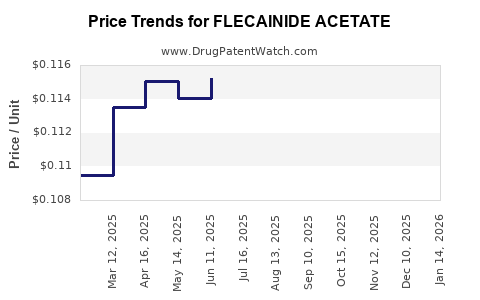
Patent expiry catalyzed generic manufacturing, precipitating a 60–70% reduction in drug prices. Generic competition has diminished profit margins for innovator companies but expanded drug accessibility. The financial trajectory now hinges on market share, pricing strategies, and regional regulations favoring or restricting generic substitution.
3. Future Revenue Projections
Analysts anticipate a plateau or slight decline in revenue in established markets due to the shift towards device-based therapies and novel drugs with improved safety profiles. However, for emerging markets and specialized indications (e.g., pediatric arrhythmias), niche growth opportunities could partially offset declines.
4. R&D and Portfolio Diversification
Pharmaceutical companies are shifting R&D focus toward personalized medicine, gene therapy, and safer antiarrhythmic agents. Flecainide acetate’s future financial viability may be affected by innovations that either complement or replace current usage, influencing investments and marketing strategies.
Emerging Trends and Opportunities
- Biomarker-Guided Therapy: Enhanced patient stratification could optimize flecainide use, potentially restoring confidence in its safety profile and expanding indications.
- Combination Regimens: Use with other agents to improve efficacy may create new market segments.
- Regulatory Approvals in Developing Countries: Growth opportunities lie in expanding approvals and manufacturing for emerging markets, where cardiovascular disease prevalence is rising.
Challenges and Risks
- Safety Concerns: Proarrhythmic risks associated with flecainide necessitate careful patient selection and limit widespread adoption.
- Market Saturation: Mature markets are highly commoditized, with low margins due to generics.
- Technological Displacement: Advancements in ablation technologies threaten long-term reliance on pharmacotherapy.
Key Takeaways
- The market for flecainide acetate is characterized by a high dependence on mature markets, with declining revenues driven by generics and competition from device therapies.
- Regulatory pressures and safety profiles are pivotal factors influencing market dynamics.
- Opportunities exist in emerging markets and niche indications, contingent on regulatory approval and clinical evidence.
- The shift toward personalized medicine and technological innovations poses both risks and opportunities for the drug’s financial trajectory.
- Companies investing in newer, safer antiarrhythmic agents may influence the future landscape of flecainide acetate’s market share.
FAQs
1. What are the main therapeutic indications for flecainide acetate?
Flecainide acetate is primarily used to treat atrial fibrillation, atrial flutter, and certain ventricular arrhythmias, especially in patients who do not respond to or cannot tolerate other therapies.
2. How have patent expirations affected the market for flecainide acetate?
Patent expirations have led to increased generic manufacturing, significantly reducing drug prices and revenues for branded formulations, while expanding access in various regions.
3. What are the safety concerns associated with flecainide acetate?
Potential proarrhythmic effects, especially in patients with structural heart disease or those prone to ischemia, limit its use and call for rigorous patient selection and monitoring.
4. How do technological advances impact the demand for flecainide acetate?
Emerging therapies like catheter ablation and advanced monitoring have reduced reliance on pharmacological management, potentially constraining market growth.
5. What strategic opportunities exist for pharmaceutical companies regarding flecainide acetate?
Expanding into emerging markets, developing combination therapies, and establishing biomarkers for safer use present avenues for growth amid competitive pressures.
References
[1] Global Burden of Disease Study. (2019). Cardiovascular diseases statistics.
[2] U.S. Food and Drug Administration. (2022). Drug Approvals and Patent Expirations.
[3] MarketWatch. (2021). Antiarrhythmic Drugs Market Revenue Analysis.