Last updated: September 4, 2025
Introduction
Etravirine, marketed under the brand name Intelence, is a non-nucleoside reverse transcriptase inhibitor (NNRTI) used in the treatment of HIV-1 infection. Approved by the U.S. Food and Drug Administration (FDA) in 2008, Etravirine has since established itself as a vital component in antiretroviral therapy (ART) regimens for HIV-positive patients. Understanding the drug's market dynamics and financial trajectory requires an analysis of its clinical positioning, competitive landscape, regulatory trends, manufacturing factors, and macroeconomic influences that shape its valuation and growth prospects.
1. Clinical Positioning and Market Demand
Etravirine's role as a second-line treatment option stems from its efficacy in patients who have developed resistance to first-generation NNRTIs such as efavirenz and nevirapine. Its unique mechanism of binding to multiple sites on the reverse transcriptase enzyme confers high resistance barriers, making it attractive in salvage therapy.
The global HIV treatment market continues to grow, driven by rising prevalence and expanded access programs. According to UNAIDS, approximately 38 million people worldwide live with HIV, with over 25 million on antiretroviral therapy as of 2021. The increasing adoption of Etravirine in low- and middle-income countries (LMICs), especially where resistance to first-line therapies is high, supports sustained demand.
However, the drug's market share has been challenged by newer agents in the NNRTI class, such as doravirine and the growing use of integrase strand transfer inhibitors (INSTIs) like dolutegravir, which are favored for their improved side effect profiles and simplified dosing regimens.
2. Competitive Landscape
Etravirine faces intense competition from multiple drug classes:
- Integrase Inhibitors: Dolutegravir (DTG) and bictegravir have gained dominance as first-line therapies due to superior efficacy and tolerability.
- Other NNRTIs: Rilpivirine remains a competitor in certain regimens, although resistance issues are a concern.
- Protease Inhibitors (PIs): Used in salvage therapy but less preferred due to adverse effects and pill burdens.
In this context, Etravirine's positioning as a secondary or salvage therapy limits its market growth potential but sustains steady demand in treatment-resistant cases. Some studies indicate residual Off-label use and preference shifts in specific regions, especially where resistance testing informs personalized therapy choices.
3. Regulatory and Patent Landscape
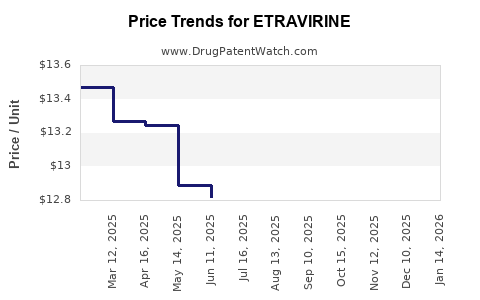
Etravirine’s patent expiration timelines crucially influence its market trajectory. The original patents in the United States expired in 2020, opening avenues for generic manufacturing, which typically results in substantial price erosion—downward pressure on revenue in developed markets. In emerging markets, patent protections often persist longer, providing a temporary shield from generic competition.
Regulatory developments also impact the drug's landscape:
- Approval of Generics: Several generics are approved in various jurisdictions, contributing to price competition.
- New Indications or Formulations: Patent extensions via new formulations or fixed-dose combinations can temporarily bolster revenue.
4. Manufacturing and Supply Chain Considerations
Manufacturing Etravirine involves complex synthetic pathways, and shifts toward generic production have scaled up capacity, significantly lowering costs. This proliferation enhances access but compresses profit margins for originator companies. Supply chain stability, particularly amid global disruptions like COVID-19, sustains affordability and availability in resource-poor settings, which remains critical for demand continuity.
5. Pricing and Reimbursement Dynamics
Pricing strategies for Etravirine vary by region:
- Developed Markets: Prices are higher, driven by patent exclusivity, with reimbursement flows through healthcare payers.
- Emerging Markets: Lower prices due to generic competition and subsidy programs.
Reimbursement policies, especially in LMICs—funded through global initiatives like Gilead’s PEPFAR, the Clinton Foundation, and the Global Fund—affect access and revenue streams. Additionally, price negotiations and health policy shifts toward combination therapies influence Etravirine's market penetration.
6. Macro-Economic and Epidemiological Influences
The ongoing HIV epidemic in lower-income regions offers long-term demand for effective salvage therapies like Etravirine. However, several macro factors bear influence:
- Funding Fluctuations: Changes in international aid impact procurement volumes.
- Generic Market Dynamics: Entry of generics in key markets will depress prices and revenues.
- Innovative Therapy Adoption: Transition toward novel drug classes may diminish Etravirine's market share over time.
7. Revenue and Financial Outlook
Initially, Etravirine recorded peak annual revenues of approximately $400–500 million in the late 2010s. Post-patent expiry in key markets and escalating generic competition have depressed sales significantly. As of 2022, global sales have declined by over 60%, with top pharmaceutical companies recalibrating their portfolios accordingly.
Despite declining revenues, Etravirine remains a relevant option within highly resistant HIV cases, especially in settings with limited access to newer agents. The drug's ongoing utility in salvage therapy sustains a niche market, albeit with diminishing financial returns.
8. Future Trajectory and Strategic Considerations
Looking ahead, the financial trajectory hinges on several factors:
- Market Penetration in Low-Income Countries: Continued reliance on Etravirine in resistance management sustains some demand.
- Emergence of New Resistance Patterns: Limited, but ongoing, resistance challenges necessitate alternative therapies.
- Development of Fixed-Dose Combinations: Integration into multi-drug formulations may rejuvenate interest, prolonging lifecycle.
Pharmaceutical firms may also focus on lifecycle management strategies, such as new dosing regimens, formulation improvements, or expanded indications, to extend profitability.
Key Market Drivers and Barriers
| Drivers |
Barriers |
| Rising global HIV prevalence |
Patent expiration and generic competition |
| High resistance prevalence necessitating salvage therapy |
Competition from newer, more tolerable agents |
| International aid funding for treatment access |
Limited penetration in developed markets post-patent expiry |
| Increasing use of fixed-dose combinations |
Stringent regulatory and reimbursement hurdles |
Conclusion
Etravirine’s market dynamics are shaped by its clinical niche, the evolving HIV treatment landscape, patent protections, and macroeconomic factors. While its revenues face pressure from generics and emerging treatment options, the drug's role in managing resistant HIV cases sustains moderate demand. Companies and stakeholders must navigate patent cliffs, price competition, and treatment landscape shifts while leveraging opportunities in targeted markets.
Key Takeaways
- Market Positioning: Etravirine remains vital for salvage therapy in resistant HIV cases, though its broader market share diminishes alongside newer agents.
- Patent and Generic Impact: Patent expiries have precipitated significant revenue declines; generics dominate prices in many regions.
- Regional Variability: Demand persists primarily in resource-limited settings where resistance testing guides therapy choices.
- Strategic Opportunities: Lifecycle management via formulation innovations and regional partnerships can extend the drug's market viability.
- Investment Implication: For stakeholders, understanding regional dynamics and resistance trends is essential for informed positioning.
FAQs
1. What factors have most contributed to the decline in Etravirine’s revenues?
Patent expirations leading to generic entry, competition from newer drug classes like INSTIs, and shifting treatment guidelines favoring simpler regimens have all contributed to revenue declines.
2. How does resistance impact the demand for Etravirine?
Etravirine’s efficacy as a salvage therapy makes it crucial for patients with resistance to other NNRTIs, sustaining demand in cases where resistance testing indicates its use.
3. Are there ongoing efforts to extend Etravirine’s patent life or develop new formulations?
While no recent patent extensions are reported, companies may explore fixed-dose combination formulations or new delivery methods to prolong lifecycle.
4. Which regions harbor the most significant market for Etravirine?
Sub-Saharan Africa and parts of Asia, where resource limitations restrict access to newer, more convenient drugs, rely more heavily on Etravirine.
5. What is the outlook for Etravirine’s role in future HIV treatment strategies?
Its role is expected to diminish in favor of first-line INSTIs and other novel agents, but it remains a crucial component for highly resistant cases in specific markets.
References
- UNAIDS. Global HIV & AIDS statistics—Fact sheet 2022.
- U.S. Food and Drug Administration. FDA approves Intelence for HIV treatment, 2008.
- MarketWatch. HIV antiretroviral drugs market analysis, 2022.
- Gilead Sciences. Global HIV treatment access programs overview, 2022.
- World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery, 2021.