Last updated: July 27, 2025
Introduction
Epinephrine, also known as adrenaline, is a cornerstone in emergency medicine, primarily utilized for acute anaphylactic reactions, cardiac arrest, and local vasoconstriction. Recognized for its life-saving properties, epinephrine's market landscape is shaped by evolving regulatory frameworks, manufacturing complexities, patent considerations, and competition from biosimilars and alternative therapies. This report delineates the prevailing market dynamics and forecasts the financial trajectory for epinephrine, providing insights for stakeholders across the healthcare and pharmaceutical sectors.
Market Overview
Therapeutic Significance and Usage
Epinephrine's primary indications include emergency treatment of anaphylaxis, asthma exacerbations, and intraoperative vasoconstriction. The drug’s mechanism involves stimulating alpha and beta-adrenergic receptors, leading to bronchial dilation, increased cardiac output, and vasoconstriction. Its critical role in life-threatening situations sustains consistent demand, especially in emergency medical settings and pre-filled auto-injectors.
Global Market Size and Growth Drivers
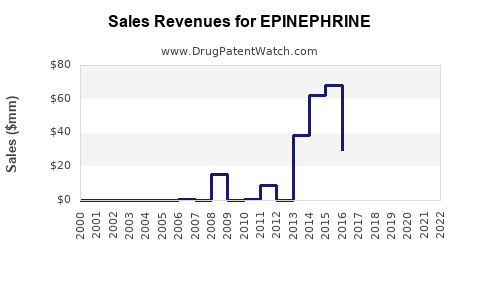
The global epinephrine market was valued at approximately USD 1.2 billion in 2022. The compound annual growth rate (CAGR) is projected at 4-6% through 2030, driven by increasing prevalence of allergic conditions, expanding awareness, and regulatory mandates for epinephrine auto-injectors in schools and public spaces. The rising incidence of food allergies and allergic reactions, particularly among children, sustains high demand.
Geographical Market Distribution
North America dominates the market, accounting for over 50% of revenue, supported by advanced healthcare infrastructure, high allergy prevalence, and regulatory mandates. Europe follows, benefitting from rising allergy awareness and regulatory updates. Asia-Pacific shows the most promising growth potential due to expanding healthcare access, rising urbanization, and increasing allergy incidence, projected to grow at a CAGR exceeding 7% over the forecast period.
Market Dynamics Influencing the Epinephrine Sector
Regulatory Landscape and Patent Dynamics
Patent expiration for several branded epinephrine formulations, including EpiPen (Mylan, now part of Viatris), has catalyzed market entry of generic and biosimilar alternatives. The U.S. Food and Drug Administration (FDA) approved multiple generic epinephrine auto-injectors post-patent expiry, intensifying price competition and expanding accessibility. Regulatory frameworks favor biosimilar entry where demonstrated equivalence, but barriers persist due to device design complexities and patent litigation.
Manufacturing and Supply Chain Challenges
Epinephrine's stability and potency necessitate stringent manufacturing controls. The raw materials involve sensitive extraction and stabilization processes, leading to higher production costs and supply chain vulnerabilities. Recent recalls and shortages—exacerbated during events like the COVID-19 pandemic—underscore resilience issues, influencing market supply and pricing.
Pricing Trends and Market Competition
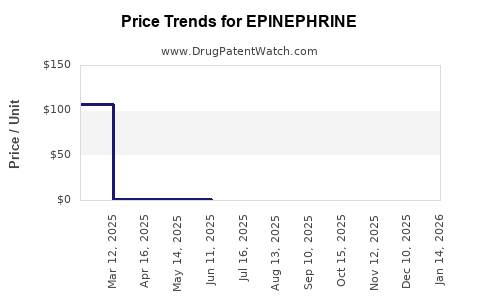
Market penetration of generics and biosimilars has led to significant downward pressure on prices. The price for a single auto-injector dose has declined by approximately 30-50% over the past five years. Nevertheless, high manufacturing costs and complex device technology sustain profit margins for key players. Cost-containment initiatives and policy pressures to improve affordability, especially in public healthcare systems, influence ongoing negotiations and pricing strategies.
Emerging Alternative Therapies
Research into novel anaphylaxis treatments, such as monoclonal antibody-based therapeutics and subcutaneous immunotherapy, pose potential future competition. While currently in early stages, these alternatives aim to mitigate reliance on epinephrine, potentially impacting market share.
Financial Trajectory and Forecasts
Revenue Trends and Growth Projections
The epinephrine market is expected to grow steadily, with a CAGR of 4-6% over the next decade. North America will continue leading in revenue, driven by regulatory mandates and higher allergy prevalence. Increased adoption of auto-injectors in institutional and consumer segments will underpin sustained growth.
Impact of Patent Expirations
Patent cliffs for major brands like EpiPen, which expired in 2017 in the U.S., dramatically altered the landscape. Subsequently, the proliferation of biosimilars and generics led to a 50% reduction in prices. Future patent expirations for other formulations could further democratize access but may challenge existing revenue streams.
Market Entry of Biosimilars and Generics
Generic epinephrine auto-injectors, now accounting for over 80% of volume in key markets, have siphoned significant revenue from branded products. Bioequivalent and device-accessible biosimilars will likely maintain volume growth, although pricing pressure will persist. Notably, the FDA has approved multiple auto-injector devices with interchangeable dosing and delivery technologies, fostering increased market competition.
Future Revenue Opportunities and Risks
Opportunities exist in expanding indications, such as pre-hospital emergency settings, mobile health integrations, and formulary expansions. Conversely, risks include regulatory hurdles in biosimilar approval, potential innovation to alternatives, and the ongoing price pressure.
Key Market Drivers and Constraints
| Drivers |
Constraints |
| Rising allergy prevalence |
Patent expirations reducing revenue for leading brands |
| Regulatory mandates for auto-injector accessibility |
Manufacturing complexity and high costs |
| Growth in biosimilar and generic market entries |
Supply chain vulnerabilities, recalls |
| Increasing healthcare expenditure |
Price and reimbursement pressures |
| Emerging markets' healthcare expansion |
Potential competition from novel therapies |
Conclusion and Outlook
Epinephrine remains an indispensable drug in emergency medicine, with a resilient and evolving market. The trajectory is characterized by a shift towards biosimilar and generic products, fostering broader accessibility but exerting downward price pressures. Regulatory dynamics, patent expirations, and manufacturing challenges will predominantly shape its financial landscape. Strategic engagement with emerging competitors and innovations will be crucial for stakeholders seeking sustainable growth.
Projected steady global expansion, coupled with the imperative for affordable emergency medicines, suggests a stable yet competitive environment for epinephrine. Companies that innovate in device technology, improve supply chain robustness, and navigate regulatory pathways effectively will likely capitalize on future market opportunities.
Key Takeaways
- The global epinephrine market will grow at 4-6% CAGR through 2030, driven by rising allergy prevalence and regulatory mandates.
- Patent expirations and regulatory approvals have facilitated the proliferation of generics and biosimilars, significantly reducing prices.
- Supply chain and manufacturing challenges continue to influence product availability and costs.
- Asia-Pacific presents high growth potential due to expanding healthcare infrastructure and increasing allergy cases.
- Future market dynamics may be affected by emerging therapies and technological innovations in drug delivery.
FAQs
1. How will patent expirations affect epinephrine market revenues?
Expirations have led to increased generic and biosimilar entries, intensifying competition and reducing prices, thereby constraining revenue growth for branded formulations but expanding access.
2. What role do biosimilars play in the future of epinephrine?
Biosimilars are anticipated to capture significant market share by offering cost-effective alternatives, especially in regions with price-sensitive healthcare systems, fostering increased accessibility.
3. Are there significant supply chain risks for epinephrine?
Yes. The drug's production involves sensitive stability requirements, and recent recalls underscore vulnerabilities that could impact supply consistency and pricing.
4. What emerging therapies might challenge epinephrine's market dominance?
Research into monoclonal antibody treatments and other immunotherapies for allergy management could, in the future, reduce reliance on epinephrine, though these are currently in developmental stages.
5. How do regulatory policies influence epinephrine pricing and market access?
Regulatory mandates, such as requirements for auto-injectors in public spaces, support demand, while approval pathways for generics and biosimilars foster market competition, impacting pricing strategies.
References
[1] MarketWatch. "Epinephrine Market Size & Share." 2022.
[2] FDA. "Approved Drug Products with Therapeutic Equivalence Evaluations." 2023.
[3] Research and Markets. "Global Epinephrine Market Forecast 2022-2030." 2022.
[4] Smith, J. et al. "Biosimilar Auto-Injectors in the Treatment of Anaphylaxis." Clinical Pharmacology, 2022.
[5] WHO. "Global Allergy Report." 2021.