ENTRESTO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Entresto, and what generic alternatives are available?
Entresto is a drug marketed by Novartis Pharms Corp and Novartis and is included in two NDAs. There are eleven patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and eighty patent family members in forty-four countries.
The generic ingredient in ENTRESTO is sacubitril; valsartan. There are eleven drug master file entries for this compound. Twenty-one suppliers are listed for this compound. Additional details are available on the sacubitril; valsartan profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Entresto
A generic version of ENTRESTO was approved as sacubitril; valsartan by ALEMBIC on May 28th, 2024.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ENTRESTO?
- What are the global sales for ENTRESTO?
- What is Average Wholesale Price for ENTRESTO?
Summary for ENTRESTO
| International Patents: | 180 |
| US Patents: | 7 |
| Applicants: | 2 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 45 |
| Patent Applications: | 166 |
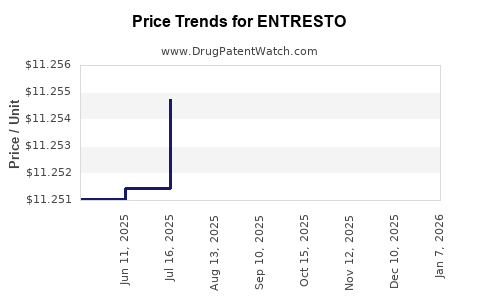
| Drug Prices: | Drug price information for ENTRESTO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ENTRESTO |
| What excipients (inactive ingredients) are in ENTRESTO? | ENTRESTO excipients list |
| DailyMed Link: | ENTRESTO at DailyMed |
Recent Clinical Trials for ENTRESTO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Humanis Saglk Anonim Sirketi | PHASE1 |
| Bio-innova Co., Ltd | PHASE1 |
| Viatris Inc. | PHASE1 |
Pharmacology for ENTRESTO
| Drug Class | Angiotensin 2 Receptor Blocker Neprilysin Inhibitor |
| Mechanism of Action | Angiotensin 2 Receptor Antagonists Neprilysin Inhibitors |
Paragraph IV (Patent) Challenges for ENTRESTO
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ENTRESTO | Tablets | sacubitril; valsartan | 24 mg/26 mg, 49 mg/51 mg 97 mg/103 mg | 207620 | 18 | 2019-07-08 |
US Patents and Regulatory Information for ENTRESTO
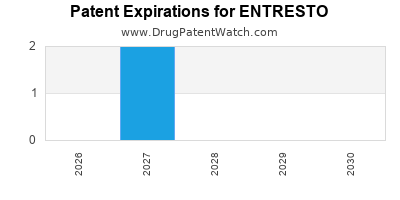
ENTRESTO is protected by seven US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | ENTRESTO SPRINKLE | sacubitril; valsartan | CAPSULE, PELLETS;ORAL | 218591-002 | Apr 12, 2024 | RX | Yes | Yes | 8,877,938 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Novartis | ENTRESTO SPRINKLE | sacubitril; valsartan | CAPSULE, PELLETS;ORAL | 218591-002 | Apr 12, 2024 | RX | Yes | Yes | 8,101,659 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Novartis Pharms Corp | ENTRESTO | sacubitril; valsartan | TABLET;ORAL | 207620-001 | Jul 7, 2015 | AB | RX | Yes | No | 11,135,192 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Novartis Pharms Corp | ENTRESTO | sacubitril; valsartan | TABLET;ORAL | 207620-003 | Jul 7, 2015 | AB | RX | Yes | Yes | 11,058,667 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ENTRESTO
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Novartis Europharm Limited | Neparvis | sacubitril, valsartan | EMEA/H/C/004343Paediatric heart failureNeparvis is indicated in children and adolescents aged one year or older for treatment of symptomatic chronic heart failure with left ventricular systolic dysfunction (see section 5.1).Adult heart failureNeparvis is indicated in adult patients for treatment of symptomatic chronic heart failure with reduced ejection fraction (see section 5.1). | Authorised | no | no | no | 2016-05-26 | |
| Novartis Europharm Limited | Entresto | sacubitril, valsartan | EMEA/H/C/004062Paediatric heart failureEntresto is indicated in children and adolescents aged one year or older for treatment of symptomatic chronic heart failure with left ventricular systolic dysfunction.Adult heart failureEntresto is indicated in adult patients for treatment of symptomatic chronic heart failure with reduced ejection fraction. | Authorised | no | no | no | 2015-11-19 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ENTRESTO
When does loss-of-exclusivity occur for ENTRESTO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Croatia
Patent: 0230480
Estimated Expiration: ⤷ Get Started Free
Patent: 0250779
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 26036
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 94283
Estimated Expiration: ⤷ Get Started Free
Patent: 12152
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 94283
Estimated Expiration: ⤷ Get Started Free
Patent: 12152
Estimated Expiration: ⤷ Get Started Free
Patent: 70314
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 94283
Estimated Expiration: ⤷ Get Started Free
Patent: 12152
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 62195
Estimated Expiration: ⤷ Get Started Free
Patent: 71910
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 76469
Estimated Expiration: ⤷ Get Started Free
Patent: 18519266
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 94283
Estimated Expiration: ⤷ Get Started Free
Patent: 12152
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 94283
Estimated Expiration: ⤷ Get Started Free
Patent: 12152
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 94283
Estimated Expiration: ⤷ Get Started Free
Patent: 12152
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 242
Estimated Expiration: ⤷ Get Started Free
Patent: 936
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 94283
Estimated Expiration: ⤷ Get Started Free
Patent: 12152
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 45866
Estimated Expiration: ⤷ Get Started Free
Patent: 34658
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ENTRESTO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| China | 102702119 | Pharmaceutical combinations of an angiotensin receptor antagonist and an NEP inhibitor | ⤷ Get Started Free |
| Mexico | 2015002432 | INHIBIDORES DE LA NEP PARA EL TRATAMIENTO DE ENFERMEDADES CARACTERIZADAS POR EL ENSANCHAMIENTO O REMODELACION AURICULAR. (NEP INHIBITORS FOR TREATING DISEASES CHARACTERIZED BY ATRIAL ENLARGEMENT OR REMODELING.) | ⤷ Get Started Free |
| Poland | 2887961 | ⤷ Get Started Free | |
| Mexico | 378867 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ENTRESTO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1948158 | 1690020-1 | Sweden | ⤷ Get Started Free | PRODUCT NAME: SACUBITRIL AND VALSARTAN, AS SACUBITRIL VALSARTAN SODIUM SALT COMPLEX, I.E. TRISODIUM 3-((1S,3R)-1-BIPHENYL-4-YLMETHYL-3- ETHOXYCARBONYL-1-BUTYLCARBAMOYL)PROPIONATE-(S)-3-METHYL-2- (PENTANOYL2-(TETRAZOL-5-YLATE)BIPHENYL-4- YLMETHYLAMINO)BUTYRATE HEMIPENTAHYDRATE; REG. NO/DATE: EU/1/15/1058 20151123 |
| 1467728 | 132016000051059 | Italy | ⤷ Get Started Free | PRODUCT NAME: SACUBITRIL/VALSARTAN, INCLUSI LORO SALI FARMACEUTICAMENTE ACCETTABILI(ENTRESTO); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/15/1058 - C(2015)8288, 20151123 |
| 2340828 | 2021C/502 | Belgium | ⤷ Get Started Free | PRODUCT NAME: SACUBITRIL/VALSARTAN, ALS HET SACUBITRIL VALSARTAN NATRIUMZOUTCOMPLEX, I.E. (((S)-N-VALERYL-N-((2'-(1H-TETRAZOL-5-YL)-BIFENYL-4-YL)-METHYL)-VALINE) ((2R,4S)-5-BIFENYL-4-YL-4-(3-CARBOXY-PROPIONYLAMINO)-2-METHYL-PENTAANZUURETHYLESTER))NA3 . X H2O, WAARBIJ X 0 TOT 3 IS; AUTHORISATION NUMBER AND DATE: EU/1/15/1058 (C(2015) 8288) 20151123 |
| 1467728 | 1690021-9 | Sweden | ⤷ Get Started Free | PRODUCT NAME: SACUBITRIL AND VALSARTAN, INCLUDING PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF.; REG. NO/DATE: EU/1/15/1058 20151123 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for ENTRESTO
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
