DULERA Drug Patent Profile
✉ Email this page to a colleague
When do Dulera patents expire, and when can generic versions of Dulera launch?
Dulera is a drug marketed by Organon Llc and is included in one NDA.
The generic ingredient in DULERA is formoterol fumarate; mometasone furoate. There are nineteen drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the formoterol fumarate; mometasone furoate profile page.
Summary for DULERA
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 4 |
| Formulation / Manufacturing: | see details |
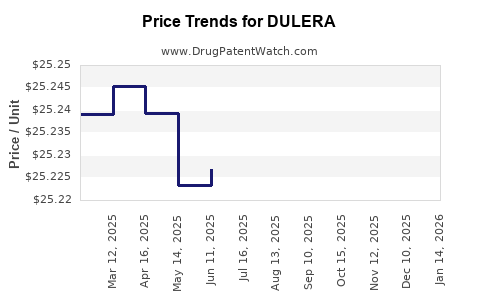
| Drug Prices: | Drug price information for DULERA |
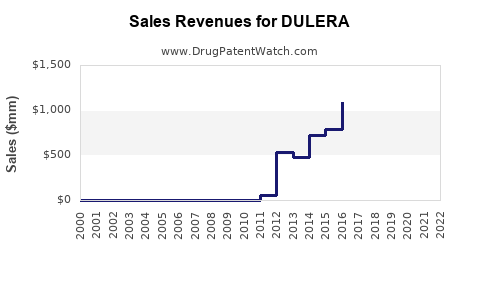
| Drug Sales Revenues: | Drug sales revenues for DULERA |
| What excipients (inactive ingredients) are in DULERA? | DULERA excipients list |
| DailyMed Link: | DULERA at DailyMed |
Recent Clinical Trials for DULERA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Sanofi | Phase 2 |
| Regeneron Pharmaceuticals | Phase 2 |
| West Penn Allegheny Health System | Phase 4 |
Pharmacology for DULERA
| Drug Class | Corticosteroid beta2-Adrenergic Agonist |
| Mechanism of Action | Adrenergic beta2-Agonists Corticosteroid Hormone Receptor Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for DULERA
US Patents and Regulatory Information for DULERA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-003 | Aug 12, 2019 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-001 | Jun 22, 2010 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-002 | Jun 22, 2010 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for DULERA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-001 | Jun 22, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-002 | Jun 22, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-002 | Jun 22, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-001 | Jun 22, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-002 | Jun 22, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-001 | Jun 22, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-002 | Jun 22, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for DULERA
See the table below for patents covering DULERA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Portugal | 740550 | ⤷ Sign Up | |
| Japan | 5646828 | ⤷ Sign Up | |
| Spain | 2200837 | ⤷ Sign Up | |
| Singapore | 47387 | Use of mometasone furoate for treating airway passage and lung diseases | ⤷ Sign Up |
| China | 1531967 | ⤷ Sign Up | |
| South Africa | 200106442 | Combinations of formoterol and mometasone furoate for asthma. | ⤷ Sign Up |
| Peru | 44995 | FUROATO DE MOMETASONA PARA EL TRATAMIENTO DE LAS ENFERMEDADES PULMONARES Y DE LAS VIAS RESPIRATORIAS | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for DULERA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2435024 | LUC00208 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: COMBINAISON DE FORMOTEROL (Y COMPRIS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES), DE GLYCOPYRRONIUM (Y COMPRIS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES) ET DE BUDESONIDE (Y COMPRIS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES); AUTHORISATION NUMBER AND DATE: EU/1/20/1468 20201210 |
| 2435024 | PA2021511,C2435024 | Lithuania | ⤷ Sign Up | PRODUCT NAME: FORMOTEROLIO (ISKAITANT BET KOKIAS FARMACINIU POZIURIU PRIIMTINAS JO DRUSKAS, ESTERIUS, SOLVATUS ARBA ENATIOMERUS), GLIKOPIROLATO (ISKAITANT BET KOKIAS FARMACINIU POZIURIU PRIIMTINAS JO DRUSKAS, ESTERIUS, SOLVATUS ARBA ENANTIOMERUS) IR BUDEZONIDO (ISKAITANT BET KOKIAS FARMACINIU POZIURIU PRIIMTINAS JO DRUSKAS, ESTERIUS, SOLVATUS ARBA ENATIOMERUS) DERINYS; REGISTRATION NO/DATE: EU/1/20/1498 20201209 |
| 2435024 | 122021000026 | Germany | ⤷ Sign Up | PRODUCT NAME: GLYCOPYRROLAT MIT FORMOTEROL MIT BUDESONID, ODER SALZE, ESTER, SOLVATE, ENANTIOMERE UND MISCHUNGEN VON ENANTIOMEREN DERSELBEN; REGISTRATION NO/DATE: EU/1/20/1498 20201209 |
| 2435024 | C202130025 | Spain | ⤷ Sign Up | PRODUCT NAME: UNA COMBINACION DE FORMOTEROL ( INCLUIDAS SUS SALES, ESTERES, SOLVATOS O ENANTIOMEROS FARMACEUTICAMENTE ACEPTABLES I, GLICOPIRROLATO ( INCLUIDAS SUS SALES, ESTERES, SOLVATOS O ENANTIOMEROS FARMACEUTICAMENTE ACEPTABLES ) Y BUDESONIDA ( INCLUIDAS SUS SALES, ESTERES, SOLVATOS O ENANTIOMEROS FARMACEUTICAMENTE ACEPTABLES ).; NATIONAL AUTHORISATION NUMBER: EU/1/20/1498; DATE OF AUTHORISATION: 20201209; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/20/1498; DATE OF FIRST AUTHORISATION IN EEA: 20201209 |
| 2435024 | 15/2021 | Austria | ⤷ Sign Up | PRODUCT NAME: FORMOTEROLFUMARAT-DIHYDRAT / GLYCOPYRRONIUMBROMID / BUDESONID; REGISTRATION NO/DATE: EU/1/20/1498 (MITTEILUNG) 20201210 |
| 2435024 | CA 2021 00014 | Denmark | ⤷ Sign Up | PRODUCT NAME: KOMBINATION AF FORMOTEROL, HERUNDER ALLE FARMACEUTISK ACCEPTABLE SALTE, ESTERE, SOLVATER ELLER ENANTIOMERE DERAF, GLYCOPYRROLAT, HERUNDER ALLE FARMACEUTISK ACCEPTABLE SALTE, ESTERE, SOLVATER ELLER ENANTIOMERE DERAF, OG BUDESONID, HERUNDER...; REG. NO/DATE: EU/1/20/1498 20201210 |
| 2435024 | 2190014-7 | Sweden | ⤷ Sign Up | PRODUCT NAME: A COMBINATION OF FORMOTEROL INCLUDING ANY PHARMACEUUTICALLY ACCEPTABLE SALTS, ESTERS, OR SOLVATES THEREOF, GLYCOPYRROLATE INCLUDING ANY PHARMACEUTICALLY ACCEPTABLE SALTS, ESTERS, OR SOLVATES THEREOF, AND BUDESONIDE INCLUDING ANY PHARMACEUTICALLY ACCEPTABLE SALT, ESTERS ORSOLVATES THEREOF; REG. NO/DATE: EU/1/20/1498 20201210 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


