DUAVEE Drug Patent Profile
✉ Email this page to a colleague
When do Duavee patents expire, and what generic alternatives are available?
Duavee is a drug marketed by Wyeth Pharms and is included in one NDA. There are two patents protecting this drug.
This drug has twenty-two patent family members in twenty countries.
The generic ingredient in DUAVEE is bazedoxifene acetate; estrogens, conjugated. There are three drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the bazedoxifene acetate; estrogens, conjugated profile page.
DrugPatentWatch® Generic Entry Outlook for Duavee
Duavee was eligible for patent challenges on October 13, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 10, 2027. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for DUAVEE
| International Patents: | 22 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 12 |
| Patent Applications: | 5 |
| Formulation / Manufacturing: | see details |
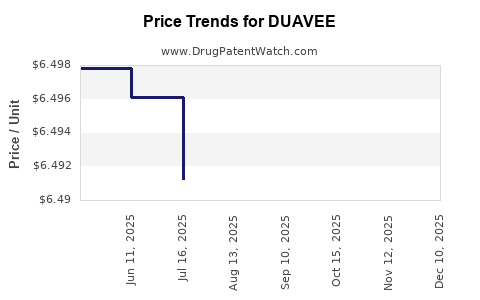
| Drug Prices: | Drug price information for DUAVEE |
| What excipients (inactive ingredients) are in DUAVEE? | DUAVEE excipients list |
| DailyMed Link: | DUAVEE at DailyMed |
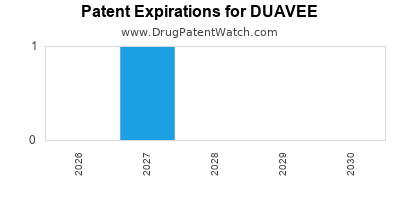
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for DUAVEE
Generic Entry Date for DUAVEE*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for DUAVEE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| VA Office of Research and Development | Phase 2 |
| The John B. Pierce Laboratory | Phase 4 |
| Penn State University | Phase 4 |
Pharmacology for DUAVEE
| Drug Class | Estrogen Agonist/Antagonist Estrogen |
| Mechanism of Action | Estrogen Receptor Agonists Selective Estrogen Receptor Modulators |
Anatomical Therapeutic Chemical (ATC) Classes for DUAVEE
US Patents and Regulatory Information for DUAVEE
DUAVEE is protected by four US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of DUAVEE is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting DUAVEE
2-phenyl-1-[4-(2-aminoethoxy)-benzyl]-indole and estrogen formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: PREVENTION OF POSTMENOPAUSAL OSTEOPOROSIS
2-phenyl-1-[4-(2-aminoethoxy)-benzyl]-indole and estrogen formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MODERATE TO SEVERE VASOMOTOR SYMPTOMS ASSOCIATED WITH MENOPAUSE
Crystalline polymorph of bazedoxifene acetate
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: PREVENTION OF POSTMENOPAUSAL OSTEOPOROSIS
Crystalline polymorph of bazedoxifene acetate
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MODERATE TO SEVERE VASOMOTOR SYMPTOMS ASSOCIATED WITH MENOPAUSE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wyeth Pharms | DUAVEE | bazedoxifene acetate; estrogens, conjugated | TABLET;ORAL | 022247-001 | Oct 3, 2013 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Wyeth Pharms | DUAVEE | bazedoxifene acetate; estrogens, conjugated | TABLET;ORAL | 022247-001 | Oct 3, 2013 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for DUAVEE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Wyeth Pharms | DUAVEE | bazedoxifene acetate; estrogens, conjugated | TABLET;ORAL | 022247-001 | Oct 3, 2013 | ⤷ Try a Trial | ⤷ Try a Trial |
| Wyeth Pharms | DUAVEE | bazedoxifene acetate; estrogens, conjugated | TABLET;ORAL | 022247-001 | Oct 3, 2013 | ⤷ Try a Trial | ⤷ Try a Trial |
| Wyeth Pharms | DUAVEE | bazedoxifene acetate; estrogens, conjugated | TABLET;ORAL | 022247-001 | Oct 3, 2013 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for DUAVEE
See the table below for patents covering DUAVEE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Taiwan | 381093 | ⤷ Try a Trial | |
| Norway | 20065052 | ⤷ Try a Trial | |
| Ecuador | SP066911 | POLIMORFO CRISTALINO DE UN ACETATO DE BAZEDOXIFENO | ⤷ Try a Trial |
| Eurasian Patent Organization | 001448 | ПРОИЗВОДНЫЕ 2-ФЕНИЛ-1- [4-(2-АМИНОЭТОКСИ)БЕНЗИЛ] ИНДОЛА В КАЧЕСТВЕ ЭСТРОГЕННЫХ АГЕНТОВ (2-PHENYL-1-[4-(2-AMINOETHOXY)-BENZYL]-INDOLE COMPOUNDS AS ESTROGENIC AGENTS) | ⤷ Try a Trial |
| Denmark | 0802183 | ⤷ Try a Trial | |
| Canada | 2203078 | 2-PHENYL-1-[4-(2-AMINOETHOXY)-BENZYL]INDOLES EN TANT QU'AGENTS OESTROGENIQUES (2-PHENYL-1-[4-(2-AMINOETHOXY)-BENZYL]INDOLES AS ESTROGENIC AGENTS) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for DUAVEE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0802183 | C00802183/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: BAZEDOXIFEN; REGISTRATION NUMBER/DATE: SWISSMEDIC 58732 12.01.2010 |
| 0802183 | 09C0048 | France | ⤷ Try a Trial | PRODUCT NAME: BAZEDOXIFENE ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE IN FRANCE: EU/1/09/511/001 DU 20090417; REGISTRATION NO/DATE AT EEC: EU/1/09/511/001-004 DU 20090417 |
| 0802183 | 300416 | Netherlands | ⤷ Try a Trial | 300416, 20170415, EXPIRES: 20220414 |
| 0802183 | 2009/028 | Ireland | ⤷ Try a Trial | PRODUCT NAME: BAZEDOXIFENE AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTRATION NO/DATE: EU/1/09/511/001-004 20090417 |
| 0802183 | PA2009007,C0802183 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: BAZEDOXIFENUM; REGISTRATION NO/DATE: EU/1/09/511/001 - EU/1/09/511/004 20090417 |
| 0802183 | CA 2009 00035 | Denmark | ⤷ Try a Trial | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


