Last updated: July 27, 2025
Introduction
Digoxin, a cardiac glycoside derived from the Digitalis plant, has been a cornerstone in the management of heart failure and atrial arrhythmias for over a century. Despite its age, this drug remains relevant owing to its unique mechanism of action, established efficacy, and diverse clinical applications. Analyzing its market dynamics and financial trajectory involves understanding its patent status, regulatory environment, competitive landscape, therapeutic positioning, and evolving healthcare trends.
Historical Context and Current Market Position
Developed in the 1930s, digoxin gained popularity for its positive inotropic effects that improve cardiac output. Although no longer under patent protection—its original patent expired decades ago—the drug continues to be widely prescribed across global markets, particularly in developing countries where cost-effectiveness is paramount. While some countries favor more contemporary agents like beta-blockers and angiotensin-converting enzyme (ACE) inhibitors, digoxin's minimal cost and well-understood profile sustain its ongoing utilization.
Market Dynamics
1. Patent and Regulatory Environment
As a generic drug, digoxin's patent expiration has led to a highly competitive market with multiple pharmaceutical companies offering bioequivalent formulations. Regulatory agencies like the U.S. Food and Drug Administration (FDA) consider digoxin an off-patent drug, fostering low barriers to entry for manufacturers and resulting in sustained price pressures. Nonetheless, innovations such as refined formulations or diagnostic tools for targeted dosing could influence future market stability.
2. Therapeutic Demand and Clinical Guidelines
The ongoing necessity for heart failure management sustains demand. Clinical guidelines from entities such as the American Heart Association (AHA) recommend digoxin as an adjunct in refractory heart failure or atrial fibrillation with a controlled ventricular rate. However, emerging evidence supports shifting away from digoxin in favor of newer agents with improved safety profiles, such as SGLT2 inhibitors, potentially constraining its growth.
3. Competitive Landscape
Despite being an established therapy, digoxin faces competition from newer medications with improved tolerability profiles and targeted mechanisms. Drugs like beta-blockers, ARNIs (angiotensin receptor-neprilysin inhibitors), and SGLT2 inhibitors are increasingly favored, impacting digoxin's market share. Yet, the low cost and extensive clinical experience preserve its relevance, especially in resource-limited settings.
4. Geographic Market Variability
Market penetration varies significantly:
- North America and Europe: Usage is more conservative, with strategic shifts towards newer agents.
- Emerging Markets: High reliance persists due to cost considerations, and the availability of low-cost generics maintains its presence.
- Asia-Pacific: Rapid urbanization and rising cardiovascular disease burden keep demand steady, although evolving treatment protocols introduce competitive pressures.
5. Manufacturing and Supply Chain Factors
Global manufacturing of generic digoxin depends largely on a handful of producers, predominantly in India and China, which account for significant supply volumes. Supply chain disruptions, regulatory inspections, or quality concerns can influence market stability. Additionally, quality assurance remains critical, particularly concerning narrow therapeutic indices and safety risks associated with digitalis toxicity.
Financial Trajectory
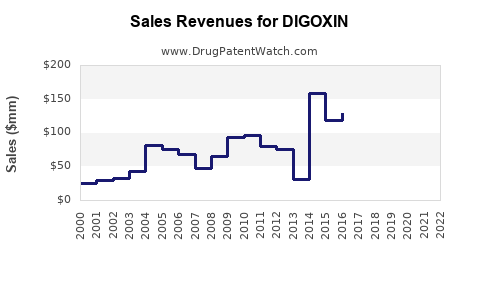
1. Revenue Trends
Given its generic status and declining prescription rates in developed markets, global revenues for digoxin are relatively flat or slowly declining. The global digitalis glycoside market is projected to experience compounded annual growth rates (CAGR) of approximately 1-2% over the next five years, driven mainly by emerging markets.
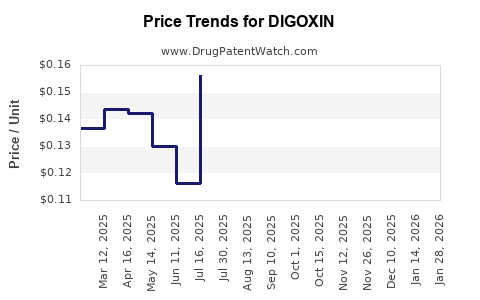
2. Pricing and Market Competition
Price erosion is prevalent due to high generic competition. In mature markets, prices for digoxin tablets have decreased significantly, with some providers offering it at a few cents per dose. Conversely, markets with limited competition or supply issues maintain higher prices temporarily.
3. R&D and Paten’s Impact
Unlike proprietary pharmaceuticals, innovation efforts for digoxin are limited. No recent patent filings or significant R&D investments are evident, focusing instead on quality control, bioequivalence, and formulation improvements to maintain market share.
4. Future Outlook
The forecast suggests a gradual decline in traditional usage in developed countries, correlating with advances in heart failure therapies. Nonetheless, in regions where cost remains a primary concern, demand persists. Governments and healthcare agencies' reimbursement policies will influence future pricing and consumption patterns.
Regulatory and Safety Considerations
Safety remains a critical factor influencing market dynamics. Digitalis toxicity risks necessitate precise dosing and monitoring, affecting prescribing practices. Regulatory bodies enforce pharmacovigilance mandates, and product labeling updates can influence market perception and physician prescribing behavior.
Emerging Trends and Market Opportunities
- Personalized Medicine: Diagnostic tools for early detection of digitalis toxicity could optimize therapy, indirectly supporting sustained use.
- Formulation Innovations: Development of extended-release formulations may improve safety and adherence, offering niche advantages.
- Digital Health Integration: Incorporation of digital monitoring devices for real-time cardiac monitoring may restore clinical confidence, potentially reviving market growth.
Conclusion: Strategic Considerations
For pharmaceutical companies and investors, digoxin offers a stable, low-margin niche within the global cardiovascular landscape. Its enduring presence hinges on factors such as healthcare policy shifts, emerging treatment paradigms, and regional demand patterns. Continued surveillance of clinical guideline updates, safety profiles, and competitive dynamics is essential for strategic planning.
Key Takeaways
- Market maturity and patent expiration position digoxin as a low-cost, high-volume generic, with slow but steady demand primarily in emerging markets.
- Evolving clinical guidelines favor newer heart failure therapies, exerting downward pressure on digoxin's market share in developed regions.
- Pricing pressures and generic competition sustain low revenue margins, but supply chain stability and quality control are critical for ongoing market presence.
- Regional variations in demand are substantial; developing markets sustain higher usage, while Europe and North America exhibit declining trends.
- Innovation avenues like advanced formulations or digital health tools may offer modest opportunities but are unlikely to reverse the overarching decline.
FAQs
1. Is digoxin still a recommended treatment in heart failure management?
Yes, in specific cases such as refractory systolic heart failure or atrial fibrillation with a rapid ventricular rate, guidelines endorse digoxin as an adjunct therapy, especially when other options are contraindicated or not tolerated.
2. How does the availability of generics affect digoxin's market?
Generic manufacturing drives down prices and increases accessibility but also intensifies competition, limiting profit margins for producers and restraining marketing efforts.
3. Are there safety concerns that could influence digoxin's future use?
Yes, digitalis toxicity poses significant safety concerns, requiring close monitoring. These risks have contributed to a gradual shift towards newer agents with wider therapeutic indices.
4. What role do emerging markets play in digoxin's future?
Emerging markets sustain demand due to affordability and limited access to newer therapies. Continued low-cost production and supply chain stability will be vital for market continuity.
5. Could innovative formulations revitalize digoxin’s market?
Potentially, extended-release formulations or digital integration could improve safety and adherence, offering niche growth opportunities, but are unlikely to substantially overturn existing market trends.
References
- American Heart Association. (2021). Guidelines for the management of heart failure.
- MarketWatch. (2022). Digitalis Glycosides Market Size, Share & Trends Analysis.
- World Health Organization. (2020). Global cardiovascular disease statistics.
- Pharmaceutical Tech. (2021). Generic Drug Market Dynamics and Trends.
- FDA. (2019). Guidance on Digitalis Drugs and Safety Monitoring.