DIFFERIN Drug Patent Profile
✉ Email this page to a colleague
When do Differin patents expire, and what generic alternatives are available?
Differin is a drug marketed by Galderma Labs Lp and is included in five NDAs. There are five patents protecting this drug and one Paragraph IV challenge.
This drug has fifty-six patent family members in twenty-two countries.
The generic ingredient in DIFFERIN is adapalene. There are twelve drug master file entries for this compound. Thirty-nine suppliers are listed for this compound. Additional details are available on the adapalene profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Differin
A generic version of DIFFERIN was approved as adapalene by P AND L on June 2nd, 2010.
Summary for DIFFERIN
| International Patents: | 56 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 5 |
| Finished Product Suppliers / Packagers: | 5 |
| Raw Ingredient (Bulk) Api Vendors: | 142 |
| Clinical Trials: | 22 |
| Patent Applications: | 4,663 |
| Formulation / Manufacturing: | see details |
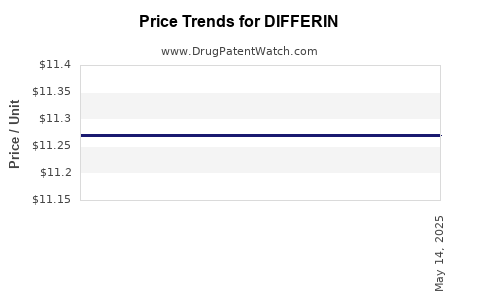
| Drug Prices: | Drug price information for DIFFERIN |
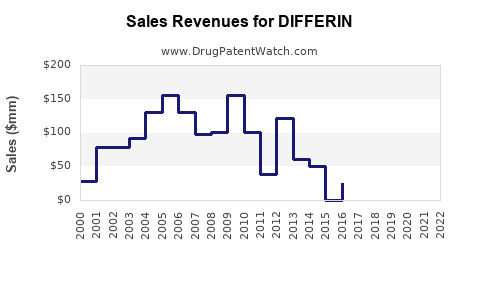
| Drug Sales Revenues: | Drug sales revenues for DIFFERIN |
| What excipients (inactive ingredients) are in DIFFERIN? | DIFFERIN excipients list |
| DailyMed Link: | DIFFERIN at DailyMed |
Recent Clinical Trials for DIFFERIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Wake Forest University Health Sciences | Early Phase 1 |
| Bausch Health Americas, Inc. | Early Phase 1 |
| Aurobindo Pharma Ltd | Phase 3 |
Pharmacology for DIFFERIN
| Drug Class | Retinoid |
Anatomical Therapeutic Chemical (ATC) Classes for DIFFERIN
US Patents and Regulatory Information for DIFFERIN
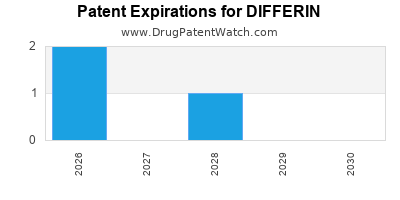
DIFFERIN is protected by five US patents.
Patents protecting DIFFERIN
Administration of 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid for the treatment of dermatological disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS
Pharmaceutical compositions comprising 0.3% by weight of 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid for the treatment of dermatological disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Cosmetic/dermatological compositions comprising naphthoic acid compounds and polyurethane polymers
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACNE
Cosmetic/dermatological compositions comprising naphtholic acid compounds and polyurethane polymers
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACNE
Cosmetic/dermatological compositions comprising naphthoic acid compounds and polyurethane polymers
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACNE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Galderma Labs Lp | DIFFERIN | adapalene | CREAM;TOPICAL | 020748-001 | May 26, 2000 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Galderma Labs Lp | DIFFERIN | adapalene | LOTION;TOPICAL | 022502-001 | Mar 17, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Galderma Labs Lp | DIFFERIN | adapalene | GEL;TOPICAL | 021753-001 | Jun 19, 2007 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Galderma Labs Lp | DIFFERIN | adapalene | SOLUTION;TOPICAL | 020338-001 | May 31, 1996 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Galderma Labs Lp | DIFFERIN | adapalene | GEL;TOPICAL | 020380-002 | Jul 8, 2016 | OTC | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for DIFFERIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Galderma Labs Lp | DIFFERIN | adapalene | GEL;TOPICAL | 021753-001 | Jun 19, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| Galderma Labs Lp | DIFFERIN | adapalene | GEL;TOPICAL | 021753-001 | Jun 19, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| Galderma Labs Lp | DIFFERIN | adapalene | CREAM;TOPICAL | 020748-001 | May 26, 2000 | ⤷ Try a Trial | ⤷ Try a Trial |
| Galderma Labs Lp | DIFFERIN | adapalene | GEL;TOPICAL | 021753-001 | Jun 19, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| Galderma Labs Lp | DIFFERIN | adapalene | SOLUTION;TOPICAL | 020338-001 | May 31, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for DIFFERIN
See the table below for patents covering DIFFERIN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Germany | 10199023 | ⤷ Try a Trial | |
| Norway | 170627 | ⤷ Try a Trial | |
| France | 2837101 | UTILISATION DE L'ACIDE 6-[1-ADAMANTYL)-4-METHOXYPHENYL]-2- NAPHTHOIQUE POUR LE TRAITEMENT DE DESORDRES DERMATOLOGIQUES | ⤷ Try a Trial |
| Norway | 861413 | ⤷ Try a Trial | |
| South Korea | 20080050579 | COMPOSITION COMPRISING AT LEAST ONE NAPHTHOIC ACID DERIVATIVE AND AT LEAST ONE COMPOUND OF POLYURETHANE POLYMER TYPE OR DERIVATIVES THEREOF, PREPARATION PROCESSES THEREFOR AND USES THEREOF | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for DIFFERIN
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1458369 | 132008901685368 | Italy | ⤷ Try a Trial | PRODUCT NAME: ADAPALENE E BENZOILE PEROSSIDO(EPIDUO); AUTHORISATION NUMBER(S) AND DATE(S): 40440, 20071218;DA 038261018/M A 038261057/M, 20080618 |
| 1458369 | 08C0024 | France | ⤷ Try a Trial | PRODUCT NAME: ADAPALENE - PEROXYDE DE BENZOLE; REGISTRATION NO/DATE IN FRANCE: NL 33724 DU 20080123; REGISTRATION NO/DATE AT EEC: 40440 DU 20071218 |
| 1458369 | SPC/GB10/005 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ADAPALENE AND BENZOYL PEROXIDE; REGISTERED: DK 40440 20071218; UK PL10590/0057 20091111 |
| 0199636 | 300209 | Netherlands | ⤷ Try a Trial | 300209, 20060411, EXPIRES: 20070702 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


