COTEMPLA XR-ODT Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Cotempla Xr-odt, and what generic alternatives are available?
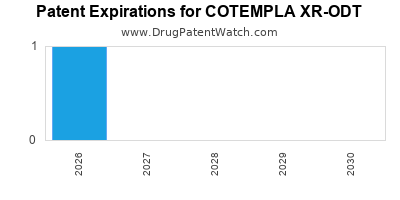
Cotempla Xr-odt is a drug marketed by Neos Theraps Inc and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has seven patent family members in five countries.
The generic ingredient in COTEMPLA XR-ODT is methylphenidate. There are thirty-two drug master file entries for this compound. Four suppliers are listed for this compound. Additional details are available on the methylphenidate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Cotempla Xr-odt
A generic version of COTEMPLA XR-ODT was approved as methylphenidate by MYLAN TECH VIATRIS on March 14th, 2022.
Summary for COTEMPLA XR-ODT
| International Patents: | 7 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 25 |
| Patent Applications: | 4,490 |
| Formulation / Manufacturing: | see details |
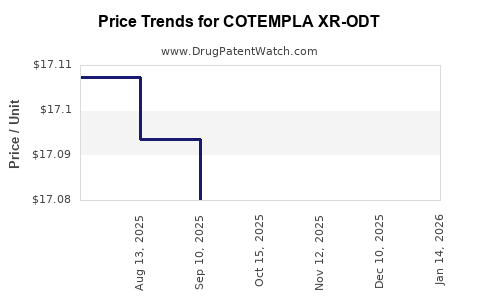
| Drug Prices: | Drug price information for COTEMPLA XR-ODT |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for COTEMPLA XR-ODT |
| What excipients (inactive ingredients) are in COTEMPLA XR-ODT? | COTEMPLA XR-ODT excipients list |
| DailyMed Link: | COTEMPLA XR-ODT at DailyMed |
Pharmacology for COTEMPLA XR-ODT
| Drug Class | Central Nervous System Stimulant |
| Physiological Effect | Central Nervous System Stimulation |
Anatomical Therapeutic Chemical (ATC) Classes for COTEMPLA XR-ODT
Paragraph IV (Patent) Challenges for COTEMPLA XR-ODT
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| COTEMPLA XR-ODT | Extended-release Orally Disintegrating Tablets | methylphenidate | 8.6 mg, 17.3 mg and 25.9 mg | 205489 | 1 | 2017-09-01 |
US Patents and Regulatory Information for COTEMPLA XR-ODT
COTEMPLA XR-ODT is protected by four US patents.
Patents protecting COTEMPLA XR-ODT
Effective dosing of a child for the treatment of ADHD with methylphenidate
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ATTENTION DEFICIT HYPERACTIVITY DISORDER (ADHD) IN PEDIATRIC PATIENTS
Compositions and methods of making rapidly dissolving ionically masked formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Compositions comprising methylphenidate complexed with ion-exchange resin particles
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Compositions comprising methylphenidate complexed with ion-exchange resin particles
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neos Theraps Inc | COTEMPLA XR-ODT | methylphenidate | TABLET, ORALLY DISINTEGRATING, EXTENDED RELEASE;ORAL | 205489-001 | Jun 19, 2017 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Neos Theraps Inc | COTEMPLA XR-ODT | methylphenidate | TABLET, ORALLY DISINTEGRATING, EXTENDED RELEASE;ORAL | 205489-002 | Jun 19, 2017 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Neos Theraps Inc | COTEMPLA XR-ODT | methylphenidate | TABLET, ORALLY DISINTEGRATING, EXTENDED RELEASE;ORAL | 205489-001 | Jun 19, 2017 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Neos Theraps Inc | COTEMPLA XR-ODT | methylphenidate | TABLET, ORALLY DISINTEGRATING, EXTENDED RELEASE;ORAL | 205489-003 | Jun 19, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Neos Theraps Inc | COTEMPLA XR-ODT | methylphenidate | TABLET, ORALLY DISINTEGRATING, EXTENDED RELEASE;ORAL | 205489-002 | Jun 19, 2017 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Neos Theraps Inc | COTEMPLA XR-ODT | methylphenidate | TABLET, ORALLY DISINTEGRATING, EXTENDED RELEASE;ORAL | 205489-001 | Jun 19, 2017 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Neos Theraps Inc | COTEMPLA XR-ODT | methylphenidate | TABLET, ORALLY DISINTEGRATING, EXTENDED RELEASE;ORAL | 205489-001 | Jun 19, 2017 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for COTEMPLA XR-ODT
When does loss-of-exclusivity occur for COTEMPLA XR-ODT?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
European Patent Office
Patent: 26066
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering COTEMPLA XR-ODT around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Australia | 2017353921 | Effective dosing of a child for the treatment of ADHD with methylphenidate | ⤷ Try a Trial |
| South Korea | 20190107655 | ADHD의 치료를 위한 메틸페니데이트의 효과적인 아동 투여 | ⤷ Try a Trial |
| Japan | 2020504763 | メチルフェニデートを用いてADHDを処置するための小児の有効な投薬 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2013003622 | ⤷ Try a Trial | |
| European Patent Office | 2726066 | FORMES POSOLOGIQUES POUR ADMINISTRATION ORALE ET MÉTHODES DE TRAITEMENT LES UTILISANT (DOSAGE FORMS FOR ORAL ADMINISTRATION AND METHODS OF TREATMENT USING THE SAME) | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2018085256 | ⤷ Try a Trial | |
| European Patent Office | 3585439 | DOSAGE EFFICACE D'UN ENFANT POUR LE TRAITEMENT DU TDAH AVEC DU MÉTHYLPHÉNIDATE (EFFECTIVE DOSING OF A CHILD FOR THE TREATMENT OF ADHD WITH METHYLPHENIDATE) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


