COMETRIQ Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Cometriq, and when can generic versions of Cometriq launch?
Cometriq is a drug marketed by Exelixis and is included in one NDA. There are eight patents protecting this drug.
This drug has one hundred and eighty-nine patent family members in thirty-one countries.
The generic ingredient in COMETRIQ is cabozantinib s-malate. There are three drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the cabozantinib s-malate profile page.
DrugPatentWatch® Generic Entry Outlook for Cometriq
Cometriq was eligible for patent challenges on November 29, 2016.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be February 10, 2032. This may change due to patent challenges or generic licensing.
There have been fourteen patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for COMETRIQ?
- What are the global sales for COMETRIQ?
- What is Average Wholesale Price for COMETRIQ?
Summary for COMETRIQ
| International Patents: | 189 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 79 |
| Clinical Trials: | 55 |
| Patent Applications: | 5,712 |
| Drug Prices: | Drug price information for COMETRIQ |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for COMETRIQ |
| What excipients (inactive ingredients) are in COMETRIQ? | COMETRIQ excipients list |
| DailyMed Link: | COMETRIQ at DailyMed |
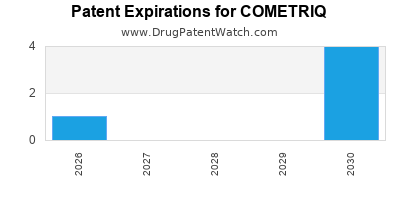

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for COMETRIQ
Generic Entry Date for COMETRIQ*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for COMETRIQ
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Cancer Institute (NCI) | Phase 2/Phase 3 |
| Children's Oncology Group | Phase 2/Phase 3 |
| Oregon Health and Science University | Phase 2 |
Pharmacology for COMETRIQ
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Protein Kinase Inhibitors |
US Patents and Regulatory Information for COMETRIQ
COMETRIQ is protected by eight US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of COMETRIQ is ⤷ Get Started Free.
This potential generic entry date is based on patent 9,717,720.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exelixis | COMETRIQ | cabozantinib s-malate | CAPSULE;ORAL | 203756-001 | Nov 29, 2012 | RX | Yes | No | 8,877,776 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Exelixis | COMETRIQ | cabozantinib s-malate | CAPSULE;ORAL | 203756-001 | Nov 29, 2012 | RX | Yes | No | 7,579,473 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Exelixis | COMETRIQ | cabozantinib s-malate | CAPSULE;ORAL | 203756-001 | Nov 29, 2012 | RX | Yes | No | 11,091,440 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Exelixis | COMETRIQ | cabozantinib s-malate | CAPSULE;ORAL | 203756-002 | Nov 29, 2012 | RX | Yes | Yes | 11,091,440 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Exelixis | COMETRIQ | cabozantinib s-malate | CAPSULE;ORAL | 203756-001 | Nov 29, 2012 | RX | Yes | No | 9,717,720 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Exelixis | COMETRIQ | cabozantinib s-malate | CAPSULE;ORAL | 203756-002 | Nov 29, 2012 | RX | Yes | Yes | 7,579,473 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for COMETRIQ
When does loss-of-exclusivity occur for COMETRIQ?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 5155
Patent: PROCESOS PARA PREPARAR COMPUESTOS DE QUINOLINA Y COMPOSICIONES FARMACEUTICAS QUE CONTIENEN DICHOS COMPUESTOS
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 12214322
Patent: Processes for preparing quinoline compounds and pharmaceutical compositions containing such compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 17204877
Patent: PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 19203745
Patent: PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 20273307
Patent: PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 22246429
Patent: PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2013020362
Patent: processos para a preparação de compostos de quinolina, compostos e combinações farmacêuticas que os contem
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 26751
Patent: PROCEDES DE SYNTHESE DE QUINOLEINES ET COMPOSITIONS PHARMACEUTIQUES LES INCLUANT (PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 3459373
Patent: Processes for preparing quinoline compounds and pharmaceutical compositions containing such compounds
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 73262
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 3513
Patent: СПОСОБЫ ПОЛУЧЕНИЯ ХИНОЛИНОВЫХ СОЕДИНЕНИЙ И ФАРМАЦЕВТИЧЕСКИХ КОМПОЗИЦИЙ, СОДЕРЖАЩИХ ТАКИЕ СОЕДИНЕНИЯ (PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 1391145
Patent: СПОСОБЫ ПОЛУЧЕНИЯ ХИНОЛИНОВЫХ СОЕДИНЕНИЙ И ФАРМАЦЕВТИЧЕСКИХ КОМПОЗИЦИЙ, СОДЕРЖАЩИХ ТАКИЕ СОЕДИНЕНИЯ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 73262
Patent: PROCÉDÉS DE SYNTHÈSE DE QUINOLÉINES ET COMPOSITIONS PHARMACEUTIQUES LES INCLUANT (PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 19498
Patent: PROCÉDÉS DE PRÉPARATION DE COMPOSÉS DE QUINOLÉINE ET COMPOSITIONS PHARMACEUTIQUES CONTENANT CES COMPOSÉS (PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Georgia, Republic of
Patent: 0217235
Patent: PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 57574
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 7848
Patent: תהליכים להכנת תרכובות קווינולין ותכשירי רוקחות המכילים תרכובות כאלה (Processes for preparting quinoline compounds and pharmaceutical compositions containing such compounds)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 13598
Estimated Expiration: ⤷ Get Started Free
Patent: 14505109
Estimated Expiration: ⤷ Get Started Free
Patent: 16188216
Patent: キノリン化合物およびそのような化合物を含有する医薬組成物の調製方法 (PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 13009116
Patent: PROCESOS PARA PREPARAR COMPUESTOS DE QUINOLINA Y COMPOSICIONES FARMACEUTICAS QUE CONTIENEN TALES COMPUESTOS. (PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 4130
Patent: Processes for preparing quinoline compounds and pharmaceutical compositions containing such compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 2808
Patent: Processes for preparing quinoline compounds and pharmaceutical compositions containing such compounds
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 73262
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 73262
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1306072
Patent: PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2030447
Estimated Expiration: ⤷ Get Started Free
Patent: 140044782
Patent: PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 190049907
Patent: 퀴놀린 화합물들의 제조 방법들 및 상기 화합물들을 함유하는 약학 조성물들 (PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 200031711
Patent: 퀴놀린 화합물들의 제조 방법들 및 상기 화합물들을 함유하는 약학 조성물들 (PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 210010671
Patent: 퀴놀린 화합물들의 제조 방법들 및 상기 화합물들을 함유하는 약학 조성물들 (PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 210147117
Patent: 퀴놀린 화합물들의 제조 방법들 및 상기 화합물들을 함유하는 약학 조성물들 (PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 230158644
Patent: 퀴놀린 화합물들의 제조 방법들 및 상기 화합물들을 함유하는 약학 조성물들 (PROCESSES FOR PREPARING QUINOLINE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS CONTAINING SUCH COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 05571
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 40509
Estimated Expiration: ⤷ Get Started Free
Patent: 1309650
Patent: Processes for preparing quinoline compounds and pharmaceutical compositions containing such compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 1706249
Patent: Processes for preparing quinoline compounds and pharmaceutical compositions containing such compounds
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering COMETRIQ around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Slovenia | 2387563 | ⤷ Get Started Free | |
| New Zealand | 712808 | Processes for preparing quinoline compounds and pharmaceutical compositions containing such compounds | ⤷ Get Started Free |
| Eurasian Patent Organization | 201400110 | ⤷ Get Started Free | |
| Japan | 6342456 | ⤷ Get Started Free | |
| Brazil | 112013020362 | ⤷ Get Started Free | |
| South Korea | 20230008268 | 암의 치료를 위한 N-(4-{〔6,7-비스(메틸옥시)퀴놀린-4-일〕옥시}페닐)-N'-(4-플루오로페닐)사이클로프로판-1,1-디카르복사미드의 말산염 및 그 결정형 (----4-----11- MALATE SALT OF N-4-[67-BISMETHYLOXYQUINOLIN-4-YL]OXYPHENYL-N'-4-FLUOROPHENYLCYCLOPROPANE-11-DICARBOXAMIDE AND CRYSTALLINE FORMS THEREOF FOR THE TREATMENT OF CANCER) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for COMETRIQ
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2213661 | C 2014 036 | Romania | ⤷ Get Started Free | PRODUCT NAME: CABOZANTINIB SI ORICE FORMA ECHIVALENTA TERAPEUTIC AACESTUIA, INCLUSIV SARURILE ACCEPT DATE OF NATIONAL AUTHORISATION: 20140321; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/890/001, EU/1/13/890/002, EU/1/13/890/003; DATE OF FIRST AUTHORISATION IN EEA: 20140321 ABILE FARMACEUTIC; NATIONAL AUTHORISATION NUMBER: EU/1/13/890/001, EU/1/13/890/002, EU/1/13/890/003; |
| 2213661 | PA2014033 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: CABOZANTINIBUM; REGISTRATION NO/DATE: EU/1/13/890/001 - EU/1/13/890/003 20140321 |
| 2213661 | 1490053-4 | Sweden | ⤷ Get Started Free | PRODUCT NAME: CABOZANTINIB AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REG. NO/DATE: EU/1/13/890 20140326 |
| 2213661 | 122014000091 | Germany | ⤷ Get Started Free | PRODUCT NAME: CABOZANTINIB UND JEDES THERAPEUTISCHE AEQUIVALENT HIERVON WIE DURCH DAS GRUNDPATENT GESCHUETZT, EINSCHLIESSLICH PHARMAZEUTISCH ANNEHMBARER SALZE; REGISTRATION NO/DATE: EU/1/13/890/001-003 20140321 |
| 2213661 | 92508 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: CABOZANTINIB ET TOUTES LES FORMES THERAPEUTIQUEMENT EQUIVALENTES QUI EN DERIVENT TELLES QUE PROTEGEES PAR LE BREVET DE BASE, Y COMPRIS LES SELS PHARMACEUTIQUEMENT ACCEPTABLES. FIRST REGISTRATION: 20140326 |
| 2213661 | C20140029 00117 | Estonia | ⤷ Get Started Free | PRODUCT NAME: KABOSANTINIIB;REG NO/DATE: K(2014)2043 (LOPLIK) 26.03.2014 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory of COMETRIQ (Tivantinib): An In-Depth Analysis
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
