COMBIGAN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Combigan, and what generic alternatives are available?
Combigan is a drug marketed by Abbvie and is included in one NDA.
The generic ingredient in COMBIGAN is brimonidine tartrate; timolol maleate. There are eleven drug master file entries for this compound. Twelve suppliers are listed for this compound. Additional details are available on the brimonidine tartrate; timolol maleate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Combigan
A generic version of COMBIGAN was approved as brimonidine tartrate; timolol maleate by SANDOZ on April 4th, 2022.
Summary for COMBIGAN
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 4 |
| Clinical Trials: | 21 |
| Patent Applications: | 56 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for COMBIGAN |
| Drug Sales Revenues: | Drug sales revenues for COMBIGAN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for COMBIGAN |
| What excipients (inactive ingredients) are in COMBIGAN? | COMBIGAN excipients list |
| DailyMed Link: | COMBIGAN at DailyMed |
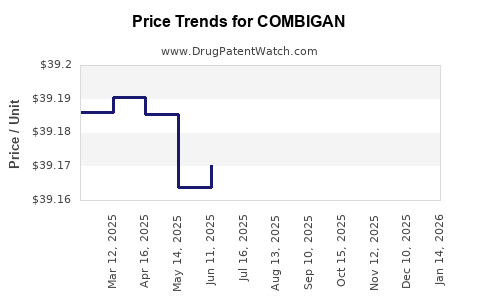
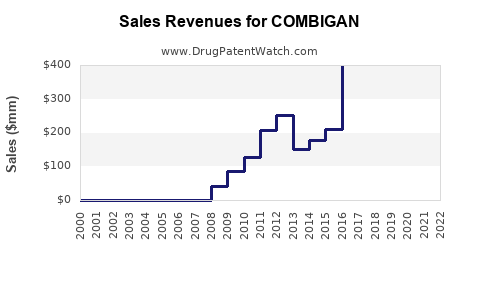
Recent Clinical Trials for COMBIGAN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Salus University | Phase 4 |
| EMS | Phase 3 |
| McMaster University | N/A |
Pharmacology for COMBIGAN
| Drug Class | alpha-Adrenergic Agonist beta-Adrenergic Blocker |
| Mechanism of Action | Adrenergic alpha-Agonists Adrenergic beta-Antagonists |
Anatomical Therapeutic Chemical (ATC) Classes for COMBIGAN
Paragraph IV (Patent) Challenges for COMBIGAN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| COMBIGAN | Ophthalmic Solution | brimonidine tartrate; timolol maleate | 0.2%/0.5% | 021398 | 1 | 2008-11-21 |
US Patents and Regulatory Information for COMBIGAN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | COMBIGAN | brimonidine tartrate; timolol maleate | SOLUTION/DROPS;OPHTHALMIC | 021398-001 | Oct 30, 2007 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for COMBIGAN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | COMBIGAN | brimonidine tartrate; timolol maleate | SOLUTION/DROPS;OPHTHALMIC | 021398-001 | Oct 30, 2007 | ⤷ Sign Up | ⤷ Sign Up |
| Abbvie | COMBIGAN | brimonidine tartrate; timolol maleate | SOLUTION/DROPS;OPHTHALMIC | 021398-001 | Oct 30, 2007 | ⤷ Sign Up | ⤷ Sign Up |
| Abbvie | COMBIGAN | brimonidine tartrate; timolol maleate | SOLUTION/DROPS;OPHTHALMIC | 021398-001 | Oct 30, 2007 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for COMBIGAN
See the table below for patents covering COMBIGAN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 1496912 | ASSOCIATION DE BRIMONIDINE ET DE TIMOLOL POUR UTILISATION OPHTALMOLOGIQUE TOPIQUE (COMBINATION OF BRIMONIDINE AND TIMOLOL FOR TOPICAL OPHTHALMIC USE) | ⤷ Sign Up |
| Portugal | 1496912 | ⤷ Sign Up | |
| Brazil | 0302584 | Combinação de brimonidina e timolol para uso oftálmico tópico | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for COMBIGAN
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1631293 | 14C0056 | France | ⤷ Sign Up | PRODUCT NAME: BRIMONIDINE OU L'UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/13/904 20140225 |
| 1631293 | 2014/041 | Ireland | ⤷ Sign Up | PRODUCT NAME: BRIMONIDINE AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTRATION NO/DATE: EU/1/13/904 20140221 |
| 1631293 | 92462 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: BRIMONIDINE ET SES SELS PHARMACEUTIQUES POUR L UTILISATION COMME MEDICAMENT POUR LE TRAITEMENT DES ROUGEURS INDUITES PAR LA ROSACEA.FIRST REGISTRATION: 20140225 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


