Last updated: July 27, 2025
rket Dynamics and Financial Trajectory for the Pharmaceutical Drug: Carvedilol
Introduction
Carvedilol, a non-selective beta-adrenergic blocker with alpha-1 blocking activity, has established itself as a cornerstone in managing cardiovascular diseases, primarily heart failure, hypertension, and post-myocardial infarction care. Approved by the FDA in 1995, its unique pharmacodynamic profile has driven steady growth, yet the evolving landscape of cardiovascular therapeutics continues to influence its market dynamics and long-term financial outlook. This analysis explores the current market environment, key drivers, competitive factors, regulatory landscape, and future financial trajectory of carvedilol.
Market Overview and Current Landscape
The global cardiovascular drug market, valued at approximately USD 55 billion in 2022, demonstrates consistent growth driven by rising cardiovascular disease prevalence. Carvedilol holds a significant niche, especially in heart failure management, with the American College of Cardiology/American Heart Association guidelines recommending its use for patients with reduced ejection fraction. Market penetration remains high in developed nations; however, emerging markets present substantial growth opportunities (Statista, 2022).
Major pharmaceutical players, including Pfizer, Mylan, and generic manufacturers, supply carvedilol, with the drug available both as brand-name formulations (e.g., Coreg by Pfizer) and generics. The patent landscape has shifted considerably since patent expiry, significantly impacting pricing and market share distribution.
Market Drivers
-
Prevalence of Cardiovascular Diseases: The increasing incidence of hypertension, heart failure, and post-MI conditions sustains high demand for carvedilol. According to the World Heart Federation, cardiovascular diseases account for approximately 17.9 million deaths annually, underpinning consistent medication use.
-
Enhanced Clinical Evidence: Robust clinical data demonstrating carvedilol’s efficacy in reducing morbidity and mortality in heart failure patients propels its adoption. The COPERNICUS trial notably established its mortality benefit, influencing treatment guidelines worldwide.
-
Generic Market Entry and Cost Dynamics: Post-patent expiry, generics have captured significant market segments, lowering costs and expanding access in low- and middle-income countries, thus broadening the potential user base.
-
Regulatory Approvals & Expanded Indications: Ongoing research exploring carvedilol’s utility in other conditions, such as sleep apnea and certain arrhythmias, could potentially expand its therapeutic indications.
Market Challenges and Constraints
-
Patent Expiry and Market Competition: Patent cliff in the early 2010s led to increased generic competition, compelling brand manufacturers to adopt aggressive pricing strategies.
-
Pricing Pressure: Cost-containment policies, especially in healthcare systems like the US Medicare program and European agencies, continue to exert downward pressure on drug prices.
-
Alternative Therapeutics: The advent of novel agents, such as angiotensin receptor-neprilysin inhibitors (ARNIs) and other beta-blockers with improved profiles, pose substitution risks.
-
Patient Compliance and Prescriber Preferences: Despite strong evidence, carvedilol’s side effect profile (e.g., hypotension, fatigue) can influence adherence, affecting overall market size.
Regulatory and Patent Landscape
Following patent expirations around 2010, multiple generic formulations entered global markets, intensifying competition. The current regulatory environment features expedited pathways for generics in emerging markets and biosimilars where applicable, impacting pricing strategies. Encapsulation of the drug’s patents in various jurisdictions and patent litigations continue to influence supply security and market exclusivity in select regions.
Financial Trajectory and Revenue Outlook
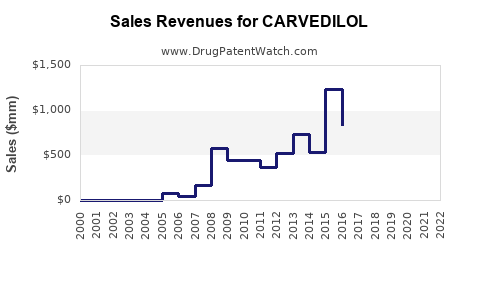
Previously, branded carvedilol products like Pfizer’s Coreg generated peak annual revenues exceeding USD 800 million globally. However, market erosion due to generics has drastically decreased these figures, with recent estimates placing global carvedilol sales around USD 150-200 million, primarily driven by developed markets where high API quality and formulation standards maintain brand loyalty.
The long-term financial outlook hinges on several factors:
-
Market Penetration in Emerging Markets: As healthcare infrastructure improves, demand is expected to rise due to increased diagnosis and treatment rates. The lower cost of generics further accelerates adoption.
-
Potential Expansion of Use Cases: Investigational studies exploring carvedilol’s role in conditions like obstructive sleep apnea or neurodegenerative diseases might open new revenue streams if positive outcomes lead to regulatory approval.
-
Product Line Extensions & Fixed-Dose Combinations: Developing combination therapies or extended-release formulations can create premium pricing opportunities and improve patient compliance, translating into revenue growth.
-
Continued Generic Expansion: The predominance of generics will likely keep unit prices low but sustain volumes, especially in price-sensitive markets. Strategic partnerships and regional manufacturing capabilities will be crucial for manufacturers to maintain competitiveness.
Forecasting and Strategic Considerations
Analysts predict carvedilol’s global revenues will stabilize over the next five years with modest growth—mainly driven by emerging economies, where cardiovascular disease burden is rising. Advanced markets may experience slight declines due to substitution by newer agents or declining prevalence of eligible patient populations as preventive measures improve.
Manufacturers investing in formulation innovations, such as fixed-dose combinations or improved bioavailability, could sustain premium segments. Furthermore, patent litigations and regulatory hurdles remain key considerations for future product development and market longevity.
Conclusion
Carvedilol remains a vital drug within the cardiovascular therapeutics landscape. Its market has evolved from dominance as a brand-name treatment to a highly competitive, predominantly generic-driven segment. Market dynamics are heavily influenced by epidemiological trends, patent expiration, cost pressures, and emerging therapeutic options.
Key Takeaways
-
Steady Demand in Developed Markets: Carvedilol continues to be prescribed extensively, especially for heart failure patients, supported by strong clinical evidence.
-
Generics Dominate Post-Patent Era: Market erosion due to generics has reduced revenues but expanded access globally.
-
Emerging Markets Offer Growth Opportunities: Rapid healthcare development and lower-cost generics position these regions as future growth drivers.
-
Innovation Needed for Differentiation: Formulation improvements and new indications could foster premium pricing and extended market lifespan.
-
Competitive Landscape Requires Strategic Response: Manufacturers must navigate price pressures, patent expiries, and increasingly competitive options to sustain revenues.
FAQs
-
What are the primary therapeutic indications for carvedilol?
Carvedilol is primarily indicated for heart failure with reduced ejection fraction, hypertension, and post-myocardial infarction management to reduce mortality and morbidity.
-
How has patent expiration affected carvedilol’s market dynamics?
Patent expirations around 2010 led to widespread availability of generics, significantly reducing prices and market share of branded formulations but expanding access, especially in emerging markets.
-
Are there any emerging therapeutic uses for carvedilol?
Current research explores carvedilol’s potential in treating sleep apnea, arrhythmias, and possibly neurodegenerative diseases, but these are not yet approved indications.
-
What factors influence carvedilol’s future revenue projections?
Key factors include demographic trends, healthcare infrastructure development in emerging markets, clinical research outcomes, regulatory policies, and competitive innovations.
-
How do new formulations or combination therapies impact carvedilol’s market?
Innovations such as fixed-dose combinations or extended-release variants could enhance patient adherence, command premium prices, and open new revenue streams.
References
[1] Statista. (2022). Global Cardiovascular Disease Market Overview.
[2] American College of Cardiology/American Heart Association guidelines. (2017). Heart Failure Management.
[3] World Heart Federation. (2022). Cardiovascular Disease Statistics.
[4] Pfizer Inc. Annual Report. (2022). Coreg Sales Data.
[5] IMS Health. (2019). Global Generic Drug Market Analysis.