BENICAR Drug Patent Profile
✉ Email this page to a colleague
When do Benicar patents expire, and what generic alternatives are available?
Benicar is a drug marketed by Cosette and is included in two NDAs.
The generic ingredient in BENICAR is hydrochlorothiazide; olmesartan medoxomil. There are thirty-two drug master file entries for this compound. Thirteen suppliers are listed for this compound. Additional details are available on the hydrochlorothiazide; olmesartan medoxomil profile page.
Summary for BENICAR
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 133 |
| Clinical Trials: | 17 |
| Patent Applications: | 4,730 |
| Formulation / Manufacturing: | see details |
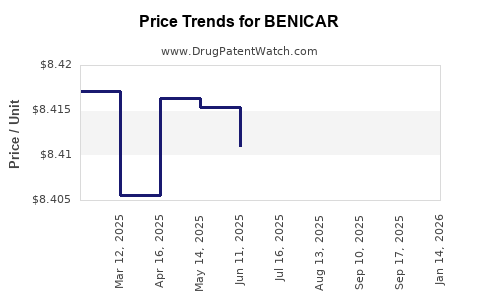
| Drug Prices: | Drug price information for BENICAR |
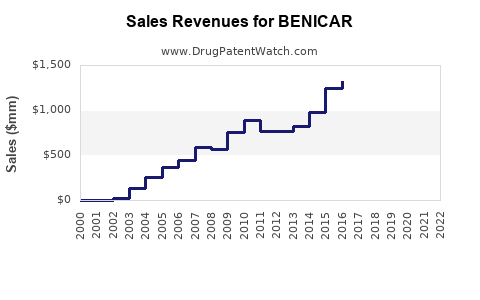
| Drug Sales Revenues: | Drug sales revenues for BENICAR |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BENICAR |
| What excipients (inactive ingredients) are in BENICAR? | BENICAR excipients list |
| DailyMed Link: | BENICAR at DailyMed |
Recent Clinical Trials for BENICAR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| EMS | Phase 3 |
| Daiichi Sankyo Co., Ltd. | N/A |
| Chinese PLA General Hospital | Phase 4 |
Pharmacology for BENICAR
| Drug Class | Angiotensin 2 Receptor Blocker |
| Mechanism of Action | Angiotensin 2 Receptor Antagonists |
Anatomical Therapeutic Chemical (ATC) Classes for BENICAR
Paragraph IV (Patent) Challenges for BENICAR
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BENICAR | Tablets | olmesartan medoxomil | 5 mg, 20 mg and 40 mg | 021286 | 1 | 2006-04-25 |
US Patents and Regulatory Information for BENICAR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-001 | Apr 25, 2002 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Cosette | BENICAR HCT | hydrochlorothiazide; olmesartan medoxomil | TABLET;ORAL | 021532-002 | Jun 5, 2003 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-003 | Apr 25, 2002 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BENICAR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-003 | Apr 25, 2002 | ⤷ Try a Trial | ⤷ Try a Trial |
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-001 | Apr 25, 2002 | ⤷ Try a Trial | ⤷ Try a Trial |
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-004 | Apr 25, 2002 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for BENICAR
See the table below for patents covering BENICAR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Spain | 2156866 | ⤷ Try a Trial | |
| Norway | 920688 | ⤷ Try a Trial | |
| Austria | 200778 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BENICAR
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0503785 | 300133 | Netherlands | ⤷ Try a Trial | 300133, 20120221, EXPIRES: 20170220 |
| 0503785 | 91056 | Luxembourg | ⤷ Try a Trial | 91056, EXPIRES: 20170221 |
| 0503785 | 20/2009 | Austria | ⤷ Try a Trial | PRODUCT NAME: KOMBINATION VON OLMESARTAN MEDOXOMIL, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH AKZEPTABLEN SALZES, MIT AMLODIPINBESILAT; NAT. REGISTRATION NO/DATE: 1-27894, 1-27895, 1-27896 20081217; FIRST REGISTRATION: NL 100989, 100990, 100991 20080819 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


