BENICAR Drug Patent Profile
✉ Email this page to a colleague
When do Benicar patents expire, and what generic alternatives are available?
Benicar is a drug marketed by Cosette and is included in two NDAs.
The generic ingredient in BENICAR is hydrochlorothiazide; olmesartan medoxomil. There are thirty-two drug master file entries for this compound. Twelve suppliers are listed for this compound. Additional details are available on the hydrochlorothiazide; olmesartan medoxomil profile page.
Summary for BENICAR
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 133 |
| Clinical Trials: | 17 |
| Patent Applications: | 4,730 |
| Formulation / Manufacturing: | see details |
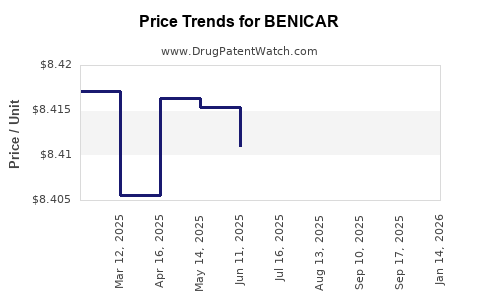
| Drug Prices: | Drug price information for BENICAR |
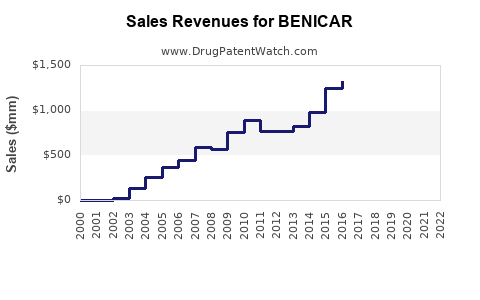
| Drug Sales Revenues: | Drug sales revenues for BENICAR |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BENICAR |
| What excipients (inactive ingredients) are in BENICAR? | BENICAR excipients list |
| DailyMed Link: | BENICAR at DailyMed |
Recent Clinical Trials for BENICAR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| EMS | Phase 3 |
| Daiichi Sankyo Co., Ltd. | N/A |
| Chinese PLA General Hospital | Phase 4 |
Pharmacology for BENICAR
| Drug Class | Angiotensin 2 Receptor Blocker |
| Mechanism of Action | Angiotensin 2 Receptor Antagonists |
Anatomical Therapeutic Chemical (ATC) Classes for BENICAR
Paragraph IV (Patent) Challenges for BENICAR
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BENICAR | Tablets | olmesartan medoxomil | 5 mg, 20 mg and 40 mg | 021286 | 1 | 2006-04-25 |
US Patents and Regulatory Information for BENICAR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-001 | Apr 25, 2002 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Cosette | BENICAR HCT | hydrochlorothiazide; olmesartan medoxomil | TABLET;ORAL | 021532-002 | Jun 5, 2003 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-003 | Apr 25, 2002 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-004 | Apr 25, 2002 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Cosette | BENICAR HCT | hydrochlorothiazide; olmesartan medoxomil | TABLET;ORAL | 021532-005 | Jun 5, 2003 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Cosette | BENICAR HCT | hydrochlorothiazide; olmesartan medoxomil | TABLET;ORAL | 021532-003 | Jun 5, 2003 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BENICAR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-003 | Apr 25, 2002 | ⤷ Sign Up | ⤷ Sign Up |
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-001 | Apr 25, 2002 | ⤷ Sign Up | ⤷ Sign Up |
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-004 | Apr 25, 2002 | ⤷ Sign Up | ⤷ Sign Up |
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-003 | Apr 25, 2002 | ⤷ Sign Up | ⤷ Sign Up |
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-001 | Apr 25, 2002 | ⤷ Sign Up | ⤷ Sign Up |
| Cosette | BENICAR | olmesartan medoxomil | TABLET;ORAL | 021286-004 | Apr 25, 2002 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for BENICAR
See the table below for patents covering BENICAR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Norway | 332938 | ⤷ Sign Up | |
| Germany | 122005000051 | ⤷ Sign Up | |
| Denmark | 0545912 | ⤷ Sign Up | |
| Russian Federation | 95101430 | ⤷ Sign Up | |
| Canada | 2097444 | DERIVES IMIDAZOLE ANTAGONISTES DE L'ANGIOTENSINE II, LEUR PREPARATION ET LEUR USAGE THERAPEUTIQUE (ANGIOTENSIN II ANTAGONIST IMIDAZOLE DERIVATIVES, THEIR PREPARATION AND THEIR THERAPEUTIC USE) | ⤷ Sign Up |
| China | 1121859 | ⤷ Sign Up | |
| World Intellectual Property Organization (WIPO) | 0241890 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BENICAR
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0503785 | C00503785/03 | Switzerland | ⤷ Sign Up | FORMER REPRESENTATIVE: BOHEST AG, CH |
| 0503785 | C300486 | Netherlands | ⤷ Sign Up | PRODUCT NAME: COMBINATIE VAN OLMESARTANMEDOXOMIL, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, AMLODIPINEBESYLAAT AND HYDROCHLOORTHIAZIDE; NATL REGISTRATION NO/DATE: RVG 106667, RVG 106671-74, RVG 106682-86 20101221; FIRST REGISTRATION: DE 79810.00.00-79814.00.00 20101216 |
| 0503785 | C300375 | Netherlands | ⤷ Sign Up | PRODUCT NAME: COMBINATIE VAN OLMESARTAN MEDOXOMIL, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT EN AMLODIPINEBESILAAT; REGISTRATION NO/DATE: RVG100984, RVG100986-87, RVG100989-91, RVG100993-95 20080819 |
| 0503785 | 91056 | Luxembourg | ⤷ Sign Up | 91056, EXPIRES: 20170221 |
| 0503785 | 9/2011 | Austria | ⤷ Sign Up | PRODUCT NAME: KOMBINATION AUS OLMESARTAN MEDOXOMIL, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH AKZEPTABLEN SALZES, AMLODIPIN BESILAT UND HYDROCHLOROTHIAZID; NAT. REGISTRATION NO/DATE: 1-30068 - 1-30072 20110216; FIRST REGISTRATION: DE 79810.00.00 - 79814.00.00, UND 79815.00.00 - 79819.00.00 20101216 |
| 0503785 | 91847 | Luxembourg | ⤷ Sign Up | 91847, EXPIRES: 20170221 |
| 0503785 | C00503785/02 | Switzerland | ⤷ Sign Up | FORMER OWNER: SANKYO COMPANY LIMITED, JP |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


