APTIOM Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Aptiom, and when can generic versions of Aptiom launch?
Aptiom is a drug marketed by Sumitomo Pharma Am and is included in one NDA. There are eleven patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred patent family members in twenty-six countries.
The generic ingredient in APTIOM is eslicarbazepine acetate. There are twelve drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the eslicarbazepine acetate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Aptiom
A generic version of APTIOM was approved as eslicarbazepine acetate by DR REDDYS on June 29th, 2021.
Summary for APTIOM
| International Patents: | 100 |
| US Patents: | 11 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 78 |
| Clinical Trials: | 3 |
| Patent Applications: | 282 |
| Formulation / Manufacturing: | see details |
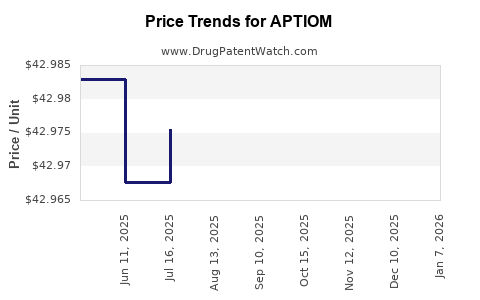
| Drug Prices: | Drug price information for APTIOM |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for APTIOM |
| What excipients (inactive ingredients) are in APTIOM? | APTIOM excipients list |
| DailyMed Link: | APTIOM at DailyMed |
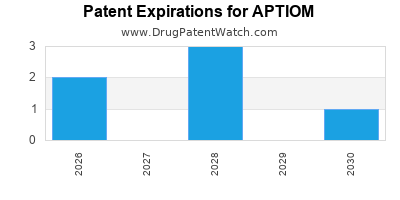
Recent Clinical Trials for APTIOM
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Sunovion | Phase 3 |
| Sunovion | Phase 4 |
| Stanford University | Phase 4 |
Pharmacology for APTIOM
| Mechanism of Action | Cytochrome P450 2C19 Inhibitors Cytochrome P450 3A4 Inducers |
| Physiological Effect | Decreased Central Nervous System Disorganized Electrical Activity |
Anatomical Therapeutic Chemical (ATC) Classes for APTIOM
Paragraph IV (Patent) Challenges for APTIOM
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| APTIOM | Tablets | eslicarbazepine acetate | 200 mg, 400 mg, 600 mg and 800 mg | 022416 | 7 | 2017-11-08 |
US Patents and Regulatory Information for APTIOM
APTIOM is protected by fifteen US patents.
Patents protecting APTIOM
Methods of treatment of partial onset seizures using eslicarbazepine acetate
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES IN A PATIENT WITH REFRACTORY PARTIAL-ONSET SEIZURES
Methods of treatment of partial onset seizures using eslicarbazepine acetate
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES
Methods of treatment of partial onset seizures using eslicarbazepine acetate
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES IN A PATIENT WITH REFRACTORY PARTIAL-ONSET SEIZURES
Methods of treatment of partial onset seizures using eslicarbazepine acetate
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES
Methods of treatment of partial onset seizures using eslicarbazepine acetate
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES
Pharmaceutical composition comprising licarbazepine acetate
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Methods of treatment of partial onset seizures using eslicarbazepine acetate
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES
Methods of treatment of partial onset seizures using eslicarbazepine acetate
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES IN A PATIENT WITH REFRACTORY PARTIAL-ONSET SEIZURES
Pharmaceutical composition comprising licarbazepine acetate
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Asymmetric catalytic reduction of oxcarbazepine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition comprising licarbazepine acetate
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Asymmetric catalytic reduction of oxcarbazepine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Treatments involving eslicarbazepine acetate or eslicarbazepine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES
Treatments involving eslicarbazepine acetate or eslicarbazepine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES IN A PATIENT SUFFERING FROM OR SUSCEPTIBLE TO ABSENCE SEIZURES
Therapeutical uses of eslicarbazepine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES IN PATIENTS WITH EPILEPSY WHO HAVE BEEN PREVIOUSLY TREATED WITH OXCARBAZEPINE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sumitomo Pharma Am | APTIOM | eslicarbazepine acetate | TABLET;ORAL | 022416-003 | Nov 8, 2013 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Sumitomo Pharma Am | APTIOM | eslicarbazepine acetate | TABLET;ORAL | 022416-003 | Nov 8, 2013 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Sumitomo Pharma Am | APTIOM | eslicarbazepine acetate | TABLET;ORAL | 022416-004 | Nov 8, 2013 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Sumitomo Pharma Am | APTIOM | eslicarbazepine acetate | TABLET;ORAL | 022416-003 | Nov 8, 2013 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Sumitomo Pharma Am | APTIOM | eslicarbazepine acetate | TABLET;ORAL | 022416-002 | Nov 8, 2013 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Sumitomo Pharma Am | APTIOM | eslicarbazepine acetate | TABLET;ORAL | 022416-002 | Nov 8, 2013 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for APTIOM
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sumitomo Pharma Am | APTIOM | eslicarbazepine acetate | TABLET;ORAL | 022416-001 | Nov 8, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| Sumitomo Pharma Am | APTIOM | eslicarbazepine acetate | TABLET;ORAL | 022416-004 | Nov 8, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| Sumitomo Pharma Am | APTIOM | eslicarbazepine acetate | TABLET;ORAL | 022416-003 | Nov 8, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| Sumitomo Pharma Am | APTIOM | eslicarbazepine acetate | TABLET;ORAL | 022416-002 | Nov 8, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for APTIOM
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| BIAL - Portela & Ca, S.A. | Zebinix | eslicarbazepine acetate | EMEA/H/C/000988 Zebinix is indicated as adjunctive therapy in adults, adolescents and children aged above 6 years, with partial-onset seizures with or without secondary generalisation. |
Authorised | no | no | no | 2009-04-21 | |
| BIAL - Portela Ca, S.A. | Exalief | eslicarbazepine acetate | EMEA/H/C/000987 Exalief is indicated as adjunctive therapy in adults with partial-onset seizures with or without secondary generalisation. |
Withdrawn | no | no | no | 2009-04-21 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for APTIOM
When does loss-of-exclusivity occur for APTIOM?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 5917
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 06273874
Estimated Expiration: ⤷ Sign Up
Austria
Patent: 20665
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0615970
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 16984
Estimated Expiration: ⤷ Sign Up
China
Patent: 1277937
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 11988
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 15346
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 15346
Estimated Expiration: ⤷ Sign Up
Patent: 19836
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 95765
Estimated Expiration: ⤷ Sign Up
Patent: 09502893
Estimated Expiration: ⤷ Sign Up
Patent: 13173764
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 08001291
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 5462
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 15346
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 3467
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 39060
Estimated Expiration: ⤷ Sign Up
Patent: 08107718
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 15346
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 0800986
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1362281
Estimated Expiration: ⤷ Sign Up
Patent: 080036212
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 71556
Estimated Expiration: ⤷ Sign Up
Patent: 62816
Estimated Expiration: ⤷ Sign Up
United Kingdom
Patent: 15690
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering APTIOM around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Russian Federation | 2008107718 | АСИММЕТРИЧЕСКОЕ КАТАЛИТИЧЕСКОЕ ВОССТАНОВЛЕНИЕ ОКСКАРБАЗЕПИНА | ⤷ Sign Up |
| South Korea | 20090110909 | Therapeutical uses of eslicarbazepine | ⤷ Sign Up |
| Spain | 2606952 | ⤷ Sign Up | |
| Mexico | 2010004323 | FORMAS DE DOSIFICACION ORAL QUE COMPRENDEN ACETATO DE LICARBAZEPINA. (ORAL DOSAGE FORMS COMPRISING LICARBAZÎ PINE ACETATE.) | ⤷ Sign Up |
| Slovenia | 2121138 | ⤷ Sign Up | |
| European Patent Office | 2319836 | Reduction catalytique asymetrique d'oxcarbazepine (Asymmetric catalytic reduction of oxcarbazepine) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for APTIOM
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1915346 | C01915346/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: ESLICARBAZEPINACETAT; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 67375 02.04.2020 |
| 0751129 | C300406 | Netherlands | ⤷ Sign Up | PRODUCT NAME: ESLICARBAZEPINE, DESGEWENST IN; REGISTRATION NO/DATE: EU/1/09/514/001-020 20090421 |
| 0751129 | SPC027/2009 | Ireland | ⤷ Sign Up | SPC027/2009: 20091119, EXPIRES: 20210627 |
| 0751129 | 360 | Finland | ⤷ Sign Up | |
| 0751129 | 09C0040 | France | ⤷ Sign Up | PRODUCT NAME: ESLICARBAZEPINE ACETATE; REGISTRATION NO/DATE IN FRANCE: EU/1/09/514/001 DU 20090421; REGISTRATION NO/DATE AT EEC: EU/1/09/514/001 DU 20090421 |
| 0751129 | SPC/GB09/047 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: ESLICARBAZEPINE ACETATE; REGISTERED: UK EU/1/09/514/001 20090421; UK EU/1/09/514/002 20090421; UK EU/1/09/514/003 20090421; UK EU/1/09/514/004 20090421; UK EU/1/09/514/005 20090421; UK EU/1/09/514/006 20090421; UK EU/1/09/514/019 20090421; UK EU/1/09/514/020 20090421; UK EU/1/09/514/013 20090421; UK EU/1/09/514/014 20090421; UK EU/1/09/514/015 20090421; UK EU/1/09/514/016 20090421; UK EU/1/09/514/017 20090421; UK EU/1/09/514/018 20090421; UK EU/1/09/514/007 20090421; UK EU/1/09/514/008 20090421; UK EU/1/09/514/009 20090421; UK EU/1/09/514/010 20090421; UK EU/1/09/514/011 20090421; UK EU/1/09/514/012 20090421 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


