APADAZ Drug Patent Profile
✉ Email this page to a colleague
When do Apadaz patents expire, and what generic alternatives are available?
Apadaz is a drug marketed by Zevra Therap and is included in one NDA. There are five patents protecting this drug.
This drug has thirty-five patent family members in twenty countries.
The generic ingredient in APADAZ is acetaminophen; benzhydrocodone hydrochloride. There are sixty-six drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the acetaminophen; benzhydrocodone hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Apadaz
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be February 22, 2031. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for APADAZ?
- What are the global sales for APADAZ?
- What is Average Wholesale Price for APADAZ?
Summary for APADAZ
| International Patents: | 35 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Drug Prices: | Drug price information for APADAZ |
| What excipients (inactive ingredients) are in APADAZ? | APADAZ excipients list |
| DailyMed Link: | APADAZ at DailyMed |
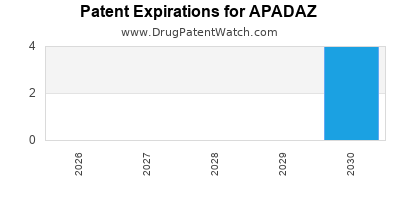

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for APADAZ
Generic Entry Date for APADAZ*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
US Patents and Regulatory Information for APADAZ
APADAZ is protected by five US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of APADAZ is ⤷ Get Started Free.
This potential generic entry date is based on patent 8,461,137.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zevra Therap | APADAZ | acetaminophen; benzhydrocodone hydrochloride | TABLET;ORAL | 208653-002 | Jan 4, 2019 | DISCN | Yes | No | 9,132,125 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Zevra Therap | APADAZ | acetaminophen; benzhydrocodone hydrochloride | TABLET;ORAL | 208653-001 | Feb 23, 2018 | DISCN | Yes | No | 9,549,923 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Zevra Therap | APADAZ | acetaminophen; benzhydrocodone hydrochloride | TABLET;ORAL | 208653-002 | Jan 4, 2019 | DISCN | Yes | No | 9,549,923 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Zevra Therap | APADAZ | acetaminophen; benzhydrocodone hydrochloride | TABLET;ORAL | 208653-003 | Jan 4, 2019 | DISCN | Yes | No | 8,828,978 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for APADAZ
When does loss-of-exclusivity occur for APADAZ?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 10266205
Patent: Benzoic acid, benzoic acid derivatives and heteroaryl carboxylic acid conjugates of hydrocodone, prodrugs, methods of making and use thereof
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2012000569
Patent: ácido benzóico, derivados de ácido benzóico e conjugados de ácido heteroaril carboxílico de hidrocodona, pró-farmacos, métodos de produção e uso destes
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 66388
Patent: ACIDE BENZOIQUE, DERIVES D'ACIDE BENZOIQUE ET CONJUGUES D'ACIDEHETEROARYLCARBOXYLIQUEE D'HYDROCODONE, PROMEDICAMENTS, LEURS PROCEDES DE FABRICATION ET D'UTILISATION (BENZOIC ACID, BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE, PRODRUGS, METHODS OF MAKING AND USE THEREOF)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 11003347
Patent: Composicion farmaceutica que comprende un conjugado, correspondiente a benzoato de hidrocodona ; y uso en el tratamiento de una enfermedad, trastorno o afeccion mediada por la union de un opioide a receptores opioides.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2480959
Patent: Benzoic acid, benzoic acid derivatives and heteroaryl carboxylic acid conjugates of hydrocodone, prodrugs, methods of making and use thereof
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 80991
Patent: ACIDO BENZOICO, DERIVADOS DE ACIDO BENZOICO Y CONJUGADOS DE ACIDO HETEROARIL CARBOXILICO DE HIDROCODONA, PROFARMACOS, METODOS PARA ELABORAR Y UTILIZAR LOS MISMOS
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 110688
Patent: CONJUGADOS DE HIDROCODONA CON ÁCIDO BENZOICO, DERIVADOS DE ÁCIDO BENZOICO Y ÁCIDO CARBOXÍLICO HETEROARÍLICO, PROFÁRCOS, MÉTODOS DE PREPARACIÓN Y USO DE LOS MISMOS
Estimated Expiration: ⤷ Get Started Free
Cuba
Patent: 128
Patent: COMPUESTO DE BENZOATO CONJUGADO A LA HIDROCODONA (BZ-HC)
Estimated Expiration: ⤷ Get Started Free
Patent: 110246
Patent: ÁCIDO BENZOICO, DERIVADOS DE ÁCIDO BENZOICO Y CONJUGADOS DE ÁCIDO HETEROARIL CARBOXÍLICO DE HIDROCODONA, PROFÁRMACOS, MÉTODOS PARA ELABORAR Y UTILIZAR LOS MISMOS
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 48407
Patent: ACIDE BENZOÏQUE, DÉRIVÉS D'ACIDE BENZOÏQUE ET CONJUGUÉS D'ACIDEHÉTÉROARYLCARBOXYLIQUEE D'HYDROCODONE, PROMÉDICAMENTS, LEURS PROCÉDÉS DE FABRICATION ET D'UTILISATION (BENZOIC ACID, BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE, PRODRUGS, METHODS OF MAKING AND USE THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 60336
Patent: ACIDE BENZOÏQUE, DÉRIVÉS D'ACIDE BENZOÏQUE ET CONJUGUÉS D'ACIDE CARBOXYLIQUE HÉTÉROARYLE D'HYDROCODONE, PROMÉDICAMENTS, PROCÉDÉS DE FABRICATION ET D'UTILISATION (BENZOIC ACID, BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE, PRODRUGS, METHODS OF MAKING AND USE THEREOF)
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 7096
Patent: תכשירים הכוללים תצמידים של בנזואט הידרוקודון ושימושים בהם (Compositions comprising conjugates of benzoate hydrocodone and use thereof)
Estimated Expiration: ⤷ Get Started Free
Patent: 9889
Patent: תצמידי חומצה בנזואית, תולדות חומצה בנזואית וחומצה קרבוקסילית הטרוארילית של הידרוקודון, מטרימי תרופות, שיטות להכנתם ושימוש בהם (Benzoic acid, benzoic acid derivatives and heteroaryl carboxylic acid conjugates of hydrocodone, prodrugs, methods of making and use thereof)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 42862
Estimated Expiration: ⤷ Get Started Free
Patent: 12532142
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 3046
Patent: BENZOIC ACID, BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE, PRODRUGS, METHODS OF MAKING AND USE THEREOF
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 12000128
Patent: ACIDO BENZOICO, DERIVADOS DE ACIDO BENZOICO Y CONJUGADOS DE ACIDO HETEROARIL CARBOXILICO DE HIDROCODONA, PROFARMACOS METODOS PARA ELABORALOS Y SUS USOS. (BENZOIC ACID, BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE, PRODRUGS, METHODS OF MAKING AND USE THEREOF.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 7235
Patent: BENZOIC ACID, BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE, PRODRUGS, METHODS OF MAKING AND USE THEREOF
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 05541
Patent: КОНЪЮГАТЫ ГИДРОКОДОНА С БЕНЗОЙНОЙ КИСЛОТОЙ, ПРОИЗВОДНЫМИ БЕНЗОЙНОЙ КИСЛОТЫ И ГЕТЕРОАРИЛКАРБОНОВОЙ КИСЛОТОЙ, ПРОЛЕКАРСТВА, СПОСОБЫ ИХ ПОЛУЧЕНИЯ И ИХ ПРИМЕНЕНИЕ (CONJUGATES OF HYDROCODONE WITH BENZOIC ACID, DERIVATIVES OF BENZOIC ACID AND HETERO-ACRYL-CARBON ACID, PRODRUGS, METHODS FOR THEIR OBTAINING AND APPLICATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 12103476
Patent: КОНЪЮГАТЫ ГИДРОКОДОНА С БЕНЗОЙНОЙ КИСЛОТОЙ, ПРОИЗВОДНЫМИ БЕНЗОЙНОЙ КИСЛОТЫ И ГЕТЕРОАРИЛКАРБОНОВОЙ КИСЛОТОЙ, ПРОЛЕКАРСТВА, СПОСОБЫ ИХ ПОЛУЧЕНИЯ И ИХ ПРИМЕНЕНИЕ
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 7445
Patent: BENZOIC ACID, BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE, PRODRUGS, METHODS OF MAKING AND USE THEREOF
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1109438
Patent: BENZOIC ACID,BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE,PRODRUGS,METHODS OF MAKING AND USE THEREOF
Estimated Expiration: ⤷ Get Started Free
Patent: 1300747
Patent: BENZOIC ACID,BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE,PRODRUGS,METHODS OF MAKING AND USE THEREOF
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1655972
Estimated Expiration: ⤷ Get Started Free
Patent: 1795330
Estimated Expiration: ⤷ Get Started Free
Patent: 1877467
Estimated Expiration: ⤷ Get Started Free
Patent: 1971223
Estimated Expiration: ⤷ Get Started Free
Patent: 2038260
Estimated Expiration: ⤷ Get Started Free
Patent: 120048589
Patent: BENZOIC ACID, BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE, PRODRUGS, METHODS OF MAKING AND USE THEREOF
Estimated Expiration: ⤷ Get Started Free
Patent: 160106781
Patent: 히드로코돈의 벤조산, 벤조산 유도체 및 헤테로아릴 카르복실산 콘쥬게이트, 이의 전구약물, 제조 방법 및 용도 (BENZOIC ACID, BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE, PRODRUGS, METHODS OF MAKING AND USE THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 170124638
Patent: 히드로코돈의 벤조산, 벤조산 유도체 및 헤테로아릴 카르복실산 콘쥬게이트, 이의 전구약물, 제조 방법 및 용도 (BENZOIC ACID BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE PRODRUGS METHODS OF MAKING AND USE THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 180081176
Patent: 히드로코돈의 벤조산, 벤조산 유도체 및 헤테로아릴 카르복실산 콘쥬게이트, 이의 전구약물, 제조 방법 및 용도 (BENZOIC ACID BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE PRODRUGS METHODS OF MAKING AND USE THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 190042115
Patent: 히드로코돈의 벤조산, 벤조산 유도체 및 헤테로아릴 카르복실산 콘쥬게이트, 이의 전구약물, 제조 방법 및 용도 (BENZOIC ACID BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE PRODRUGS METHODS OF MAKING AND USE THEREOF)
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 2916
Patent: КОМПОЗИЦИЯ НА ОСНОВЕ КОНЬЮГАТА ГИДРОКОДОНА С БЕНЗОЛЬНОЙ КИСЛОТОЙ, ПРОИЗВОДНЫМИ БЕНЗОЙНОЙ КИСЛОТ ИЛИ ГЕТЕРОАРИЛКАРБОНОВОЙ КИСЛОТОЙ, ПРОЛЕКАРСТВА, СПОСОБ ЛЕЧЕНИЯ ОТ ЗЛОУПОТРЕБЛЕНИЙ;КОМПОЗИЦІЯ НА ОСНОВІ КОН'ЮГАТУ ГІДРОКОДОНУ З БЕНЗОЙНОЮ КИСЛОТОЮ, ПОХІДНИМИ БЕНЗОЙНОЇ КИСЛОТИ АБО ГЕТЕРОАРИЛКАРБОНОВОЮ КИСЛОТОЮ, ПРОЛІКИ, СПОСІБ ЛІКУВАННЯ ВІД ЗЛОВЖИВАНЬ (COMPOSITION BASED ON CONJUGATES OF HYDROCODONE WITH BENZOIC ACID, BENZOIC ACID OR HETEROARYL CARBOXYLIC ACID DERIVATIVES, PRODRUGS AND METHOD FOR TREATMENT OF ABUSES)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering APADAZ around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20160106781 | 히드로코돈의 벤조산, 벤조산 유도체 및 헤테로아릴 카르복실산 콘쥬게이트, 이의 전구약물, 제조 방법 및 용도 (BENZOIC ACID, BENZOIC ACID DERIVATIVES AND HETEROARYL CARBOXYLIC ACID CONJUGATES OF HYDROCODONE, PRODRUGS, METHODS OF MAKING AND USE THEREOF) | ⤷ Get Started Free |
| Cuba | 20110246 | ⤷ Get Started Free | |
| European Patent Office | 2448407 | ⤷ Get Started Free | |
| South Korea | 101795330 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for APADAZ
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
