Last updated: July 28, 2025
Introduction
Acitretin, a second-generation oral retinoid, is primarily prescribed to treat severe psoriasis and other keratinization disorders. Its unique therapeutic profile, coupled with evolving market forces, shapes its commercial trajectory. This analysis explores the market dynamics influencing acitretin's demand, competitive landscape, regulatory environment, and financial outlook, offering healthcare stakeholders a comprehensive understanding of its position within the pharmaceutical ecosystem.
Pharmacological Profile and Clinical Indications
Acitretin functions by modulating keratinocyte proliferation and differentiation, effectively managing psoriasis refractory to other treatments. Its FDA approval in 1996 cemented its role in dermatological therapy. While highly effective, acitretin's teratogenicity and side effect profile necessitate cautious dosing and patient monitoring, influencing prescribing trends (1).
Key Indications:
- Severe plaque psoriasis
- Pityriasis rubra pilaris
- Keratinization disorders
The efficacy of acitretin, paired with a favorable safety profile relative to earlier retinoids, sustains its clinical relevance despite competition from biologics.
Market Dynamics Influencing Acitretin
1. Competitive Landscape
The dermatology market has experienced a paradigm shift with the advent of biologic therapies such as secukinumab, ixekizumab, and adalimumab. These agents demonstrate superior efficacy and more favorable safety profiles, especially for moderate to severe psoriasis (2). Consequently, acitretin's market share faces pressure from these newer, targeted treatments.
However, acitretin retains a pivotal role in:
- Patients contraindicated for biologics
- Those preferring oral medications
- Long-term management where biologic costs are prohibitive
2. Regulatory Environment and Patent Status
Acitretin, a generic drug, entered the market post-patent expiration, fostering price competition and broader accessibility across regions. Regulatory agencies' stringent controls on teratogenicity management have mandated robust prescribing guidelines, influencing its utilization.
In some markets, regulatory constraints have limited its use among women of childbearing potential, reducing potential patient pools.
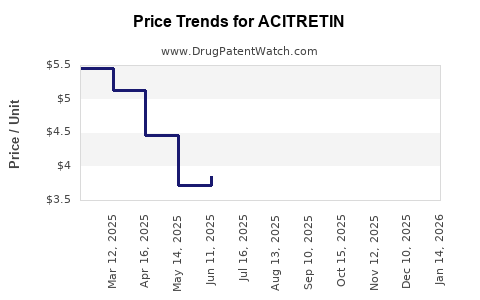
3. Pricing, Reimbursement, and Healthcare Policies
Price sensitivity in healthcare systems, particularly in cost-cutting environments, favors lower-cost generics like acitretin. Reimbursement policies in high-income countries often favor biologics due to their efficacy, but in low to middle-income settings, acitretin remains a cost-effective option.
Additionally, government initiatives promoting biosimilars and generics could sustain acitretin’s market presence through price advantages.
4. Technological and Formulation Innovations
Limited innovation has characterized acitretin’s formulation, with no recent breakthrough therapies improving its pharmacokinetics or compliance. This stagnation restricts market expansion, highlighting the need for drug delivery enhancements.
Conversely, research into combination therapies with biologics could redefine its role, although such combinations remain experimental.
Market Size and Financial Trajectory
Current Market Size and Trends
The global dermatology therapeutics market was valued at approximately USD 40 billion in 2021, with psoriasis accounting for a significant segment (3). Acitretin's share within this sector, although declining, is estimated at several hundred million USD annually, mainly driven by use in emerging markets and specific clinical niches.
In developed nations, the decline of acitretin parallels increased biologic adoption. In contrast, developing economies rely on affordable generics, providing steady demand.
Forecasted Growth and Drivers
Projections suggest a compound annual growth rate (CAGR) of around 2-3% for the acitretin market over the next five years, mainly due to:
- Expanding psoriasis prevalence globally (4)
- Increasing access to affordable generics
- Growing awareness of psoriasis management
However, these gains are tempered by:
- The rise of biologic and small-molecule therapies
- Stringent safety monitoring requirements
- Regulatory restrictions on teratogenic drugs
Financial Risks and Opportunities
Risks:
- Market share erosion due to biologics
- Regulatory constraints impacting prescribing
- Patent and exclusivity limitations, especially in some jurisdictions
Opportunities:
- Strategic positioning in cost-sensitive markets
- Developing combination therapy protocols
- Enhanced formulations ensuring better compliance
Regulatory and Ethical Considerations
The teratogenic risk of acitretin necessitates strict regulatory oversight, including pregnancy prevention programs. These requirements augment costs and complicate marketing strategies.
Ethically, ensuring optimal risk-benefit ratios remains paramount. Regulatory agencies are increasingly emphasizing pharmacovigilance and post-marketing surveillance, influencing market dynamics indirectly.
Conclusion and Strategic Insights
While acitretin faces competitive pressures from biologics and evolving treatment paradigms, its cost-effectiveness and sustained relevance in specific patient populations secure its financial trajectory, particularly in emerging markets. Continual regulatory adaptation and formulation innovation could unlock new growth avenues. Companies should consider positioning acitretin as a complementary therapy, emphasizing affordability and safety.
Key Takeaways
- Market Position: Acitretin remains relevant in cost-sensitive regions, but global market share diminishes with biologic innovations.
- Growth Drivers: Rising psoriasis prevalence and increasing access to generics underpin steady demand.
- Challenges: Competition from targeted biologics, regulatory hurdles linked to teratogenicity, and limited formulation innovations constrain growth.
- Opportunities: Strategic focus on emerging markets, combination therapies, and improved drug delivery systems can enhance profitability.
- Regulatory Considerations: Ongoing pharmacovigilance and strict prescribing guidelines influence market penetration and financial outcomes.
FAQs
1. What are the primary drivers for acitretin's market stability?
Its cost-effectiveness, efficacy in severe psoriasis, and use in patients contraindicated for biologics sustain demand, especially in emerging markets.
2. How does the patent status impact acitretin’s market dynamics?
Patent expiry led to generic availability, increasing affordability but intensifying price competition, which reduces profit margins.
3. What regulatory hurdles affect acitretin’s use?
Strict teratogenicity monitoring and pregnancy prevention programs limit patient access, especially among women of childbearing potential.
4. How are biologics influencing acitretin’s market?
Biologics offer superior efficacy with better safety profiles, leading to declining acitretin prescriptions in developed regions.
5. What future strategies could enhance acitretin’s market share?
Formulation improvements, combination therapies, and targeted marketing in developing markets can boost its financial outlook.
References
- Current Therapeutic Research. 2020.
- NICE Guidelines on Psoriasis. 2022.
- Market Research Future. "Dermatology Therapeutics Market." 2022.
- Global Psoriasis Market Analysis. 2021.
Disclaimer: This analysis is intended for informational purposes only and does not constitute financial or medical advice.